Functional analysis of interaction sites on the N-terminal domain of clathrin heavy chain (original) (raw)
. Author manuscript; available in PMC: 2012 Jul 1.
Abstract
In clathrin-mediated membrane traffic, clathrin does not bind directly to cargo and instead binds to adaptors that mediate this function. For endocytosis, the main adaptor is the AP-2 complex but it is uncertain how clathrin contacts AP-2. Here, we tested in human cells the importance of the three binding sites that have been identified so far on the N-terminal domain (NTD) of clathrin. We find that mutation of each of the three sites on the NTD, alone or in combination, does not block clathrin/AP-2-mediated endocytosis in the same way as deletion of the NTD. We report here the fourth and final site on the NTD that is required for clathrin/AP-2-mediated endocytic function. Each of the four interaction sites can operate alone to mediate endocytosis. The observed functional redundancy between interaction sites on the NTD explains how productivity of clathrin-coated vesicle formation is ensured.
Keywords: adaptors, AP-2, beta-propeller, clathrin, clathrin-box motifs, endocytosis, transferrin, vesicle
Clathrin-mediated membrane traffic governs many diverse cellular functions, from nutrient uptake and synaptic vesicle recycling to organelle biogenesis and function (1, 2). Clathrin does not bind the membrane or cargo directly (3). Instead, adaptor proteins bind to cargo and membrane; clathrin in turn, binds to the adaptor (4). Assembled clathrin coats visualized by cryo-electron microscopy reveal an outer shell that is composed of clathrin and an inner shell comprising adaptors (5-7). In these maps, the N-terminal domain (NTD) of the clathrin heavy chain (CHC) projects toward the adaptor layer implicating it as the site for clathrin/adaptor interactions (4).
In clathrin-mediated endocytosis (CME), the main adaptor is the heterotetrameric AP-2 complex (8). Other clathrin-associated sorting proteins such as the arrestins or dab2/ARH are used in concert with AP-2 for internalization of G protein-coupled receptors or LDL receptors, respectively (2, 9). The NTD of CHC is a seven-bladed beta-propeller (10, 11) and three interaction sites on the NTD have been detailed so far. First, clathrin-box motifs (CBMs) of the form LΦXΦ[DE] (single amino acid code, where Φ is a bulky hydrophobic residue and X is any amino acid, but is often polar) bind the NTD in a groove located between blades 1 and 2 (10, 12, 13). Second, the “W-box” motifs (PWXXW) bind the NTD but do not compete with CBMs (14). The binding site for W-box motifs is located at the centre of the NTD, 23 Å away from the CBM site (15). Third, a splice variant of arrestin-2, arrestin-2L, indicates that a third type of motif, [LI][LI]GXL (a2L motif), can bind to the NTD at a site between blades 4 and 5 (16).
During CME, which of these three sites is important for interaction with AP-2? It is the beta2 subunit of AP-2 that contacts clathrin (4) and for many years, it was thought that this interaction occurred solely via a CBM in the hinge region of beta2-adaptin (17). This idea was based largely on an extrapolation of an in vitro interaction detected between the beta3a subunit of AP-3 and clathrin (18). The linear motif SLLDLD in beta3a-adaptin that was necessary for clathrin-binding was similar to the LIEFE motif in arrestin-2 that is needed for binding the NTD (19) at the CBM site (13). Alignments showed that these CBMs occur in numerous adaptors and accessory proteins, including beta2-adaptin (20). Structures of the NTD in complex with peptides derived from arrestin-2 and beta3-adaptin that contain CBMs cemented the view that the CBM in beta2-adaptin must also bind at the same site during CME (12). However, several observations now indicate that this is an oversimplification. Mutations to the CBM site on the NTD that block the interaction with arrestin-2 have little effect on binding of AP-2 by the NTD (13) and deletion of the CBM from beta2-adaptin does not prevent clathrin-binding (21, 22). This indicates that other sites on beta2-adaptin may contact clathrin. Indeed, the beta2-adaptin appendage domain alone can bind clathrin (23, 24), an interaction proposed to be via a site outside of the NTD in the “ankle region” of CHC (25). Finally, the interaction between clathrin and AP-2 is central to endocytosis (2, 26), yet a scheme where this must occur via a CBM would place this important interaction at risk from competition by all the other endocytic proteins that apparently use CBMs to also contact clathrin (20).
Attempts to address the question of how clathrin and AP-2 interact during CME have relied heavily on in vitro binding experiments. However, a recent study tested the importance of the CBM site and the W-box site in yeast chc1p and found that both sites are redundant (27). The NTD of yeast chc1p is itself required for clathrin function, suggesting that additional sites in the NTD may be operational and arguing against a role for a site outside of the NTD (27). Endocytosis in yeast is different from CME in human cells (28). For example, chc1p does not bind to the AP-2-related complex in yeast and also the deletion of all adaptors has little effect on clathrin function (29). Moreover, the subsequent discovery of the a2L site on the NTD and the presence of an a2L motif in the beta2-adaptin linker (16) prompted us to test in human cells the importance for endocytosis of the various interaction sites on clathrin’s NTD.
Results
Three interaction sites identified previously on the NTD show functional redundancy
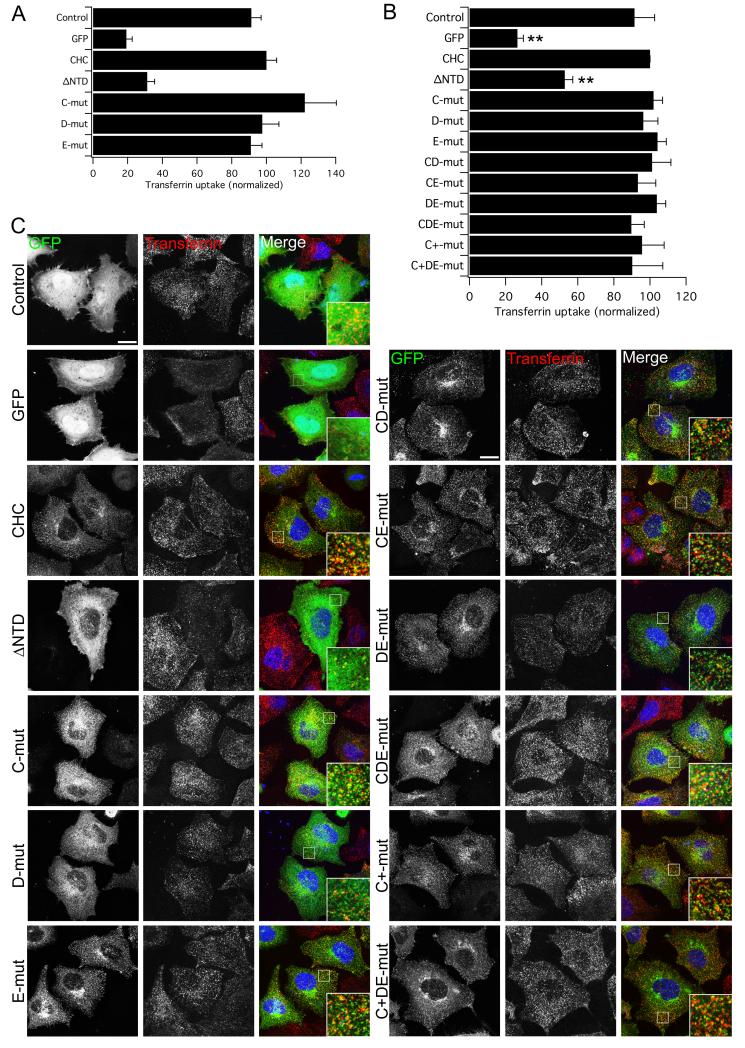
We have previously made use of a vector system to simultaneously knockdown endogenous CHC and express GFP-tagged knockdown-proof CHC constructs (30-32). This allows us to assess the effect of mutations of CHC on the function of clathrin in endocytosis. The controls for this type of experiment are GFP expressed on either a control (Control) or CHC RNAi (GFP) background and a positive control for rescue: GFP-CHC( 1-1675)KDP expressed on a CHC RNAi background (CHC). We began by testing NTD mutants in clathrin-depleted HEK293 cells using this system and examining the uptake of transferrin by confocal microscopy. The transferrin receptor is a major cargo of clathrin-coated vesicles (33) and its internalization is entirely dependent on clathrin/AP-2 (8, 34, 35). As described previously, deletion of the NTD (removal of aa 1-330, ΔNTD, see Table 1 for details) inhibited endocytosis substantially (32). However, GFP-CHC constructs that harboured mutations in the CBM site (T87A, Q89A; mutant C), the W-box site (Q152L, I154Q; mutant D) or the a2L motif site (R188A, Q192A; mutant E) were competent for endocytosis (Fig. 1A). This was surprising, given that these mutations are sufficient to inhibit binding to CBMs, W-boxes and a2L in vitro (13, 15, 16). We therefore questioned our approach and, in order to increase the number of cells analyzed, switched to flow cytometry to quantify transferrin uptake in HeLa cells (see Methods).
Table 1.
Mutations in the clathrin heavy chain and the binding sites that they target.
| Construct | Description | Details |
|---|---|---|
| ΔNTD | Removal of NTD | 331-1639 |
| C | Mutation of CBM site | T87A, Q89A |
| D | Mutation of W-box site | Q152L, I154Q |
| E | Mutation of a2L site | R188A, Q192A |
| C+ | Extra mutation of CBM site | T87A, Q89A, K96E, K98E |
| F | Mutation of putative site | N296A, R297E |
| G | Mutation of putative (fourth) site | E11K |
| G′ | Alternative mutation of fourth site | Q14D, Q16M, N17S |
| YNTD | Yeast NTD fused to CHC | chc1p(1-298)CHC(292-1675) |
| HYNTD | 1-100 aa of CHC in YNTD | CHC(1-100)chc1p(101-297)CHC(292-1675) |
| YHNTD | 1-100 aa of chc1p in CHC | chc1p(1-100)CHC(100-1675) |
Figure 1. Clathrin lacking binding sites for clathrin-box, W-box and a2L motifs is functional for clathrin/AP-2-mediated endocytosis.
A) Rescue of transferrin uptake by CHC with single-site mutations. Bar chart of transferrin uptake in HEK293 cells, normalized to CHC, measured by confocal microscopy. Ncell = 29-87, Nexp = 2. B-C) Rescue of transferrin uptake by CHC with single-, double- or triple-site mutations. B) Bar charts of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 4 (5 for C+ and C+DE). **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. In all figures, ‘Control’ is GFP expressed on a control RNAi background and all other constructs are expressed in CHC RNAi cells. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
Using this method, we could again see clear inhibition of transferrin endocytosis following clathrin-depletion and a rescue of CME with full-length CHC. These experiments confirmed that mutation of either the CBM site, the W-box site or the a2L motif site did not interfere with rescue of endocytosis of transferrin by CHC (Fig. 1B, C). CHCs harboring these mutations that were paired in any combination (mutants CD, CE, DE) or a CHC with all three sites mutated (mutant CDE) also showed functional rescue of endocytosis (Fig. 1B, C). By confocal microscopy, we could see significant transferrin uptake and also that some transferrin puncta could clearly be seen to co-localize with the clathrin constructs (Fig. 1C), indicating internalization was via clathrin-coated vesicles comprising our mutants rather than via a non-clathrin pathway. Importantly, expression of ΔNTD showed that endocytosis was significantly inhibited (Fig. 1B, C). The mean transferrin uptake for ΔNTD was not significantly different to that for GFP in all experiments in this paper (p > 0.05). These results suggested that other sites exist on the NTD that mediate clathrin/AP-2-mediated endocytosis in the absence of the binding sites identified previously.
Highlighting an important functional region of NTD using yeast chc1p
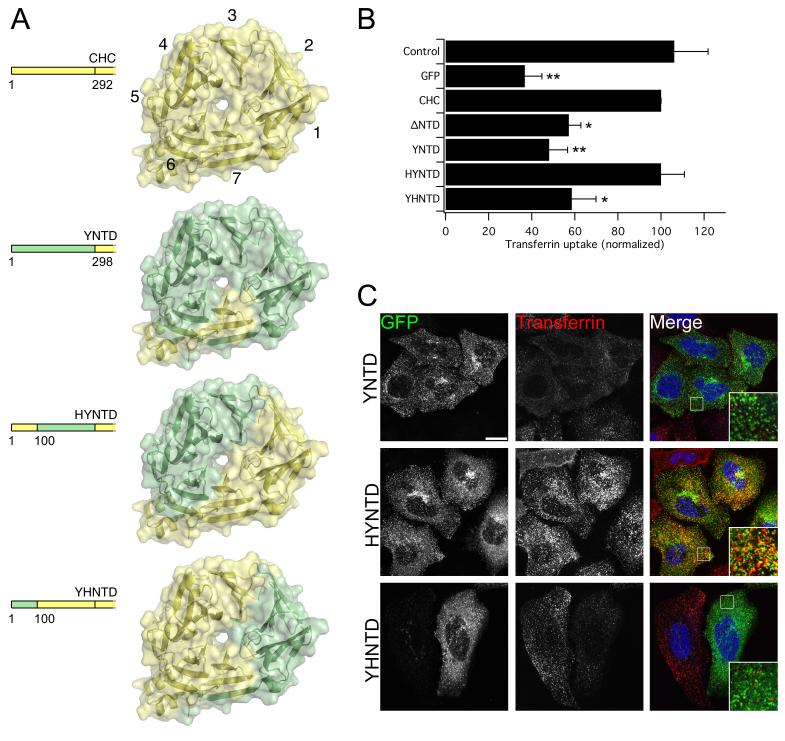
We set out to identify alternative sites and began by testing whether the yeast NTD could function in place of human NTD in HeLa cells. It has been assumed that the binding sites on the NTD are conserved from yeast to man (13). All key residues for these interactions are indeed conserved (12, 15, 16), however the AP-2-related complex in yeast cannot bind chc1p and none of the adaptors are essential for clathrin-mediated functions (29). We generated a CHC where almost the entire NTD is exchanged with that from yeast (YNTD, Fig. 2A). This mutant was unable to rescue transferrin uptake in clathrin-depleted cells (Fig. 2B, C). The expression of this construct was normal and its localization did not suggest that it was misfolded (see below). This result was unexpected as key residues of all three sites are present in YNTD and yet endocytosis cannot proceed in human cells. Importantly, the YNTD construct presented the opportunity to test which regions of the NTD are required for clathrin/AP-2-mediated endocytosis. To do this, we next generated two more chimeric CHCs: HYNTD, where the first 100 residues of human NTD were recalled into the YNTD construct; and YHNTD, in which the first 100 residues of yeast NTD were substituted into CHC (Fig. 2A). We found that HYNTD could rescue endocytosis whereas YHNTD was unable to do so(Fig. 2B, C). Again the chimeric constructs both appeared to be expressed normally (see below), leading us to conclude that residues 1-100 of human NTD but not yeast NTD were sufficient for clathrin/AP-2-mediated endocytosis in human cells.
Figure 2. The first 100 residues of human CHC are sufficient for clathrin/AP-2-mediated endocytosis.
A) Schematic illustration of yeast-human chimeric CHCs (residues 1-330). Human and yeast clathrin are colored pale yellow and green, respectively. Beta-propeller blades are numbered for CHC to aid orientation. B-C) CHC with NTD from chc1p cannot rescue transferrin uptake and uptake depends on residues 1-100. B) Bar charts of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 3. *, p <0.05. **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing chimeric GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
The first 100 residues of CHC constitute strand 7d and the entirety of blades 1 and 2. In the context of HYNTD the functional, human, surface of the NTD is from blade 6 through to blade 2 (Fig 2A), so what lies in this region? The CBM binding site lies wholly within the first 100 residues of the NTD (12) and as a fragment, residues 1-100 can completely account for CBM interactions (10, 13). As previous models dictated that AP-2 contacts the NTD via a CBM in the hinge region of beta2-adaptin, we questioned whether the mutations we had made in mutant C were insufficient in blocking the interaction with CBMs. We therefore made further mutations (K96E, K98E; mutant C+) to mutant C and to the triple mutant (CDE) to give mutants C+ and C+DE. Both of these constructs were able to rescue clathrin/AP-2-mediated endocytosis (Fig. 1A, B). These results further argue that functional clathrin/AP-2 interactions do not absolutely require the CBM site. In addition, these data rule out blades 1 and 2 from the functional region that we identified above.
Identification of the fourth and final interaction site assisted by bioinformatics
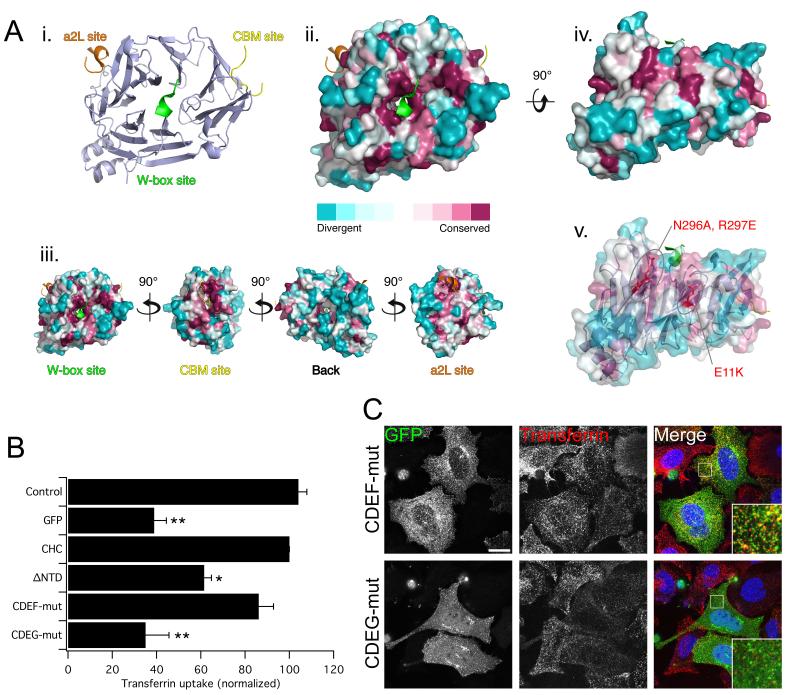
To identify other potential interaction sites, we turned to an in silico method. All of the three sites that have been identified thus far fall into regions of the NTD that are conserved (Fig. 3A). We reasoned that any further interaction sites might also lie in regions that are similarly conserved. A 3D model of the NTD (PDB 1UTC) was generated where conserved residues were mapped onto the surface using CONSURF (36). This showed that the existing sites lie in conserved patches and that the “bottom side” of the NTD, facing the leg (11), was most variable (Fig. 3A). This gave us confidence that conserved patches had the potential to be interaction sites. Two distinctive patches of conserved residues were found between blades 6 and 7, i.e. part of the functional surface of the NTD. These patches lie approximately 120° from the CBM site and 240° from the a2L site and thus give an attractive 3-fold symmetry to the binding sites relative to one another. We therefore designed two mutants to target these patches: N296A, R297E on blade 6 (mutation F) and E11K on blade 7 (mutation G). Given the apparent redundancy that we had already observed with single-site mutants, we introduced these mutations directly into the triple mutant CDE (to give CDEF, CDEG) in order to maximize our chances of seeing an effect. Mutant CDEF was able to rescue endocytosis similarly to wild-type CHC and is therefore not part of a new site. Mutant CDEG on the other hand, did not rescue endocytosis (Fig. 3B, C). By microscopy, the distribution of this mutant was significantly different to the other constructs with fewer puncta and more diffuse cytosolic fluorescence (Fig. 3C and discussed later). The transferrin uptake results suggest that E11, but not N296 and R297, is part of a fourth binding site that is required for functional clathrin/AP-2-mediated endocytosis, at least when the three established sites are unavailable for interaction. As CDEG blocked endocytosis completely, we conclude that these four regions represent the full complement of interaction sites on the NTD.
Figure 3. A fourth interaction site on the NTD, defined by sequence conservation, is required for clathrin/AP-2-mediated endocytosis.
A) Evolutionary conservation of residues on the surface of the NTD of CHC. (i) Ribbon diagram to show the approximate positions of the three binding sites (PDB codes = 1UTC, 1C9I and 3GD1). (ii) Molecular surface of the NTD with projected sequence conservation colored from cyan (divergent) to maroon (conserved). Ligands are removed in this panel. (iii) Binding sites fall within conserved patches and the back face of the NTD is more divergent. (iv) View of two conserved patches between on blades 6 and 7 suggestive of a new interaction site. (v) Design of two new mutants to target the putative interaction site. B-C) Rescue of transferrin uptake by a triple-site mutant CHC (CDE) with additional mutations at N296A, R297E (CDEF) and at E11K (CDEG). B) Bar charts of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 4. *, p <0.05. **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
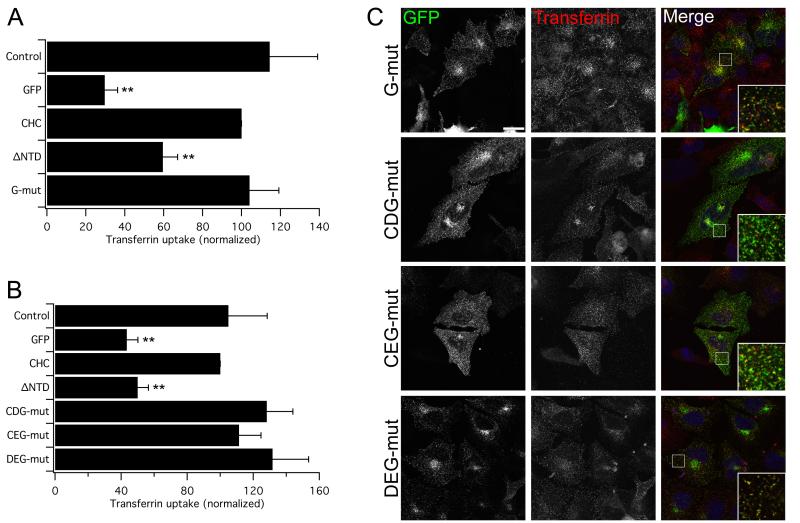
The CDEG mutant was the first combination of mutations in human CHC that resulted in blockade of endocytosis. This raised two important possibilities. First, the lack of rescue with CDEG could be due the E11K mutation resulting in misfolded clathrin. Although this is unlikely because E11 is a surface-exposed residue and so its mutation would not be expected to significantly alter the structure of the NTD. Second, as we had seen rescue with CDE but not with CDEG it is possible that the new site is the only functional site on the NTD, with the other three sites not being used at all. Both of these possibilities could be tested with a CHC harboring the E11K mutation alone. A CHC with the G mutation alone was able to rescue clathrin/AP-2-mediated endocytosis in clathrin-depleted cells (Fig. 4A, C). This result indicates that the lack of rescue in the CDEG mutant is not due to a destructive effect of the G mutation. Furthermore, it shows that one or more of the other three sites can support endocytosis after mutation of the new, fourth site.
Figure 4. The E11K mutation does not affect clathrin stability and the four interaction sites can each mediate endocytosis alone.
A) Rescue of transferrin uptake by a CHC with a single E11K mutation (G). B) Rescue of transferrin uptake by CHCs with wild type binding sites for a2L motifs (CDG), W-box motifs (CEG) and CBMs (DEG) alone. The functionality of the fourth site is shown in Fig 1 (CDE). Bar charts of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 3. **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
Each of the four interaction sites on the NTD can mediate endocytosis
The functionality of the triple mutant CDE, when compared to the non-functional CDEG, effectively demonstrates that the fourth site is operational (Figs 1 and 3). We realized that we could therefore assess the functionality of the a2L, W-box, and CBM binding sites individually by making the following triple-site mutants CDG, CEG and DEG, i.e. individually reverting each site in the CDEG mutant to the wild type residues. We found that CDG, CEG and DEG could all rescue endocytosis in clathrin-depleted cells (Fig 4B, C). So clathrin/AP-2-mediated endocytosis can proceed when clathrin can only bind a2L motifs, W-box or CBMs, respectively. As these mutants were functional despite harboring the G mutation, this is further evidence that the lack of rescue in CDEG is not due to a non-specific effect of the G mutation. Together, these results demonstrate the functional redundancy of the four interaction sites on the NTD: no single site is essential for endocytosis and each is operational on its own.
Further definition of fourth interaction site
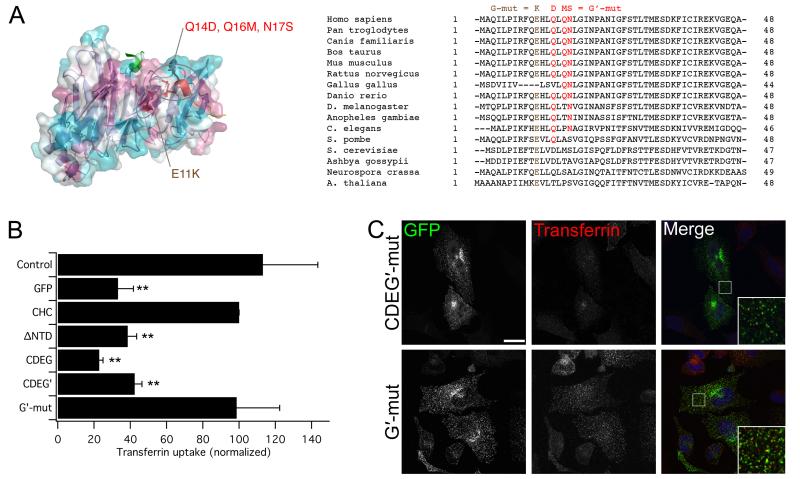
It was somewhat paradoxical that we were able to use evolutionary conservation as a tool to find the new, fourth site, given that we had earlier demonstrated a functional non-equivalence of the yeast and human NTDs (Fig. 2). Residue E11 is conserved in yeast and so we hypothesized that it is the surrounding divergent residues that result in an inactive site in YNTD and YHNTD (Fig. 5A). To test this hypothesis and to define the fourth site further, we mutated three neighboring residues to their yeast equivalents Q14D, Q16M, N17S (G′ mutation). When these point mutations were introduced into the CDE mutant (to give CDEG′) this CHC, like CDEG, was unable to rescue endocytosis in clathrin-depleted cells (Fig. 5B, C). Importantly, the G′ mutation alone did not affect functional rescue of CHC, which suggests that again these point mutations inactivate the site without affecting the overall stability of CHC (Fig. 5B, C). These results indicate that the fourth site is inactive in yeast due to the surrounding residues. In addition, as the new site could be targeted with independent mutations (G′ vs G) this strengthens our conclusion that we have identified the fourth and final site on the NTD and inactivated it by mutations that do not affect the stability of clathrin overall.
Figure 5. Further definition of the fourth interaction site by new inactivating mutations.
A) Designing a new inactivating mutation of the fourth site. View of the fourth site (as seen in Fig 3A) showing the new G′ mutation (red) the previous E11K (G mutation) is shown in brown. A sequence alignment is shown for CHCs from different organisms (right). The G′ mutation alters the human residues at positions 14, 16 and 17 to their chc1p counterparts. B) Lack of rescue of transferrin uptake by a CHC with CDEG′ and CDEG. CHC with the G′ mutation alone is functional. Bar chart of transferrin uptake in HeLa cells, normalized to CHC, measured by flow cytometry. Bars are mean ± s.e.m. Nexp = 3. **, p <0.01. C) Representative confocal micrographs of transferrin uptake (middle, red in merge) in HeLa cells expressing GFP-CHCs (left, green in merge) that were depleted of endogenous clathrin. Insets: 4X zoom of the boxed area. Scale bar, 20 μm.
Subcellular distribution of clathrin mutants
Clathrin has a familiar subcellular distribution (37). It is found in pits and vesicles throughout the cell and accumulates on vesicles and membranes at the TGN. Of all the constructs studied here, only ΔNTD and CDEG had a subcellular distribution that was noticeably different to endogenous clathrin (Figs 1-5). All constructs including ΔNTD and CDEG showed an accumulation at a perinuclear compartment (Figs 1-5) this compartment was confirmed as the TGN by co-staining with anti-TGN46 (Supplementary Fig S1). We also tested whether or not the CHC constructs co-localized with AP-2 by immunostaining (Supplementary Fig S2). We expected to see a correlation between co-localization and functionality. This was because we assumed that mutations that interfere with endocytic function should also disrupt recruitment of clathrin to AP-2-positive patches on the membrane. However, only the ΔNTD and CDEG constructs showed a significant disruption (Supplementary Fig S2). All of the chimeric yeast-human constructs co-localized with AP-2 patches in spite of a lack of functionality of YNTD and YHNTD. Moreover, CDEG′ was also found in AP-2-positive patches. These qualitative observations were verified by image correlation analysis (38) (Supplementary Fig. S2B).
These results argue that the presence of E11 is crucial for recruitment to AP-2 patches. If it is mutated, and no other interaction sites are available, then clathrin can no longer localize to AP-2 patches. The recent identification of pioneer proteins whose recruitment precedes that of AP-2 and clathrin (39) raises the possibility that clathrin could be recruited via these molecules rather than by AP-2 in an obligatory manner. E11 may be important for this interaction. Together these results indicate that the non-functionality of ΔNTD and CDEG is for a different reason than for YNTD, YHNTD and CDEG′. The latter constructs can be recruited to AP-2 patches but their presence is non-productive. In contrast, ΔNTD and CDEG are not recruited completely to AP-2 puncta and so are unable to initiate endocytosis.
Discussion
The aim of this study was to analyze in human cells the endocytic functionality of each interaction site on clathrin’s NTD. In summary, we found that the three interaction sites identified so far are not essential for endocytosis. A new site was subsequently identified via a convoluted approach. All four sites show significant functional redundancy such that mutation of all sites was necessary to ablate function.
Taking our flow cytometry results as a whole, we found only two classes of clathrin mutant: those capable of transferrin uptake and those that were not (sixteen functional versus five non-functional). These results were supported by microscopy, where ‘functional’ mutants were clearly able to uptake transferrin and those in the ‘non-functional’ group were not. While we are confident of the assignment of mutants to either of these two groups, it is possible that differences exist within these two broad groups but that these were not detected under our experimental conditions. Firstly, a single condition was used for uptake experiments: one concentration and one timepoint. Use of other experimental conditions may reveal subtle differences between the clathrin mutants that we have classed here as functional. Secondly, our analysis of the total amount of transferrin taken up into cells may overlook variation in the way the mutants internalize a similar amount of cargo, e.g. the size and rate of CCV formation. Future analysis of the dynamics of these mutants by total internal reflection fluorescence microscopy could reveal such variation. Thirdly, our conclusions and assignment of mutants are both limited to uptake of transferrin, a process known to be AP-2 dependent (8, 34, 35). Internalization of other receptor types that use alternative adaptors may reveal additional differences between the CHC mutants; perhaps in the way that uptake occurs and/or in the redundancy of sites observed.
Despite our two-hit protocol for RNAi of CHC, there is a trace amount of endogenous clathrin similarly left in all conditions and our comparisons are made against this background level (see Methods). This would not be a concern for CHCs that were completely active or inactive, but could be problematic for mutants that might be partly functional. The remaining endogenous clathrin could prevent us from resolving partly functional from fully functional CHCs. This is not likely to occur as a result of mixed triskelia as there is good evidence that transfected CHCs do not co-trimerize with endogenous clathrin (40, 41). Each experiment we performed had four control conditions that enabled us to identify presence or absence of functional rescue and to account for background clathrin. This indicates that in our system, CHC mutants form homogenous triskelia that can operate independently of the trace amounts of endogenous clathrin. However, due to the co-operative nature of clathrin assembly (42), it is possible that small amounts of endogenous clathrin could seed a CCP and mutant triskelia could then complete CCV formation. Even if this were the case, mutant triskelia would still require some functionality in order to do this, because of the observed lack of rescue in five mutant CHCs despite presumably having the same levels of endogenous clathrin. Distinguishing between fully and partly functional CHCs will be difficult using human cells without altering the endogenous CHC by gene editing (43). Our interpretation of results is supported at least in part by the work of Collette et al. (27). In this paper, endogenous clathrin was not a concern and they still found that mutation of the CBM or W-box sites does not interfere with clathrin function. In spite of the limitations discussed here, it has been possible for us to classify the CHCs into broad functional or non-functional categories and for us to use this to infer which sites on the NTD are important for clathrin/AP-2-mediated endocytosis.
Original models of CME held that the NTD contacted AP-2 exclusively via the CBM site (17). We found that, in human cells, functional clathrin/AP-2 interactions do not absolutely require the CBM site. This is not unexpected as several lines of evidence indicate that the contact between clathrin and AP-2 is not so simple. Examples include: binding experiments that demonstrate that mutation of the CBM site interferes with arrestin-2 binding but has little effect on AP-2 binding (13) and that deletion of the CBM from beta2-adaptin has little effect on clathrin binding (24) and function (22). Recently, “pitstop” compounds have been developed that bind with low affinity to the CBM site (44). The authors convincingly showed that these drugs inhibited binding of CBMs to the NTD in vitro. They also showed that pitstop 2 was able to block transferrin uptake in cells (44). Our results suggest that in cells these compounds may not act solely at the CBM site and that they may able to block all four interaction sites on the NTD and/or act elsewhere on clathrin to inhibit assembly.
Our experiments using triple-site mutants suggested that each of the four sites on the NTD is capable of mediating endocytosis. Perhaps the most interesting of these was CEG: a CHC that rescued clathrin/AP-2 mediated endocytosis via the W-box binding site alone. The W-box motif is only found in sorting nexin 9 and amphiphysins (15, 27) but is absent from AP-2. This raises the intriguing possibility that the endocytic network is ‘re-routed’ in clathrin-depleted cells expressing the CEG mutant such that AP-2 can contact clathrin via amphiphysins or SNX9 when direct contact is not possible. Under normal conditions in the cell, it is unlikely that all other sites will be occupied (on all three NTDs – on every triskelion) to leave only the W-box site free for ‘re-routing’ to occur. More likely, the contact between clathrin and AP-2 in the cell is not a simple, single line as drawn in network diagrams (26) but a blend of direct and indirect contacts. This observation highlights the plasticity of the endocytic network and the failsafe mechanisms that have evolved to ensure that this important cellular process can persist.
This study uncovered a new, fourth interaction site on the NTD of CHC. What is the nature of this fourth site? The G and G′ mutations confirm that the fourth interaction site encompasses the end of strand d of blade 7 and the helical segment in the loop connecting blades 7 and 1 (Fig 5A). This site, like the other three, must be able to bind short peptide motifs; the identity of these motifs is currently unknown. Recent work (45) suggests that clathrin-binding sequences could be more degenerate than previously recognized (9). We speculate that the fourth site could bind to CBMs, a2L motifs or other related sequences such as DLL or DLF (46). An idea that has yet to be explored is whether there could be promiscuity in the interactions of these type motifs with the sites around the rim of the NTD. If this were the case, it would be predicted that W-box motifs would bind exclusively at their defined site. Whatever the identity of the motif for the fourth site, we propose that such peptides are likely to bind between blades 6 and 7, rather than between blades 7 and 1, as this space is occupied by the helical segment (10). Two other conspicuous yeast-divergent residues in this region are R8E and H12L (Fig 5A). If this proposal were correct then we would expect that H12 would not be involved in peptide-binding but that R8 may interact if the peptide extends far enough along blade 7. Only a crystal structure of the NTD with a peptide bound at the fourth site will allow us to resolve the precise residues involved.
To conclude, this work has shown that the binding sites identified previously by binding and crystallographic experiments do not represent the full complement of interaction sites on the NTD. We found a new site situated around E11 on blade 7 of the NTD that is responsible for endocytosis in the absence of the other three sites. This fourth and final site completes the array of interaction sites on the NTD and provides important insight into how the endocytic network operates. Clathrin and AP-2 are hubs in the endocytic network and their interaction is essential to successful internalization of a variety of receptors and ligands (2, 26). If this interaction occurred exclusively via CBMs, then competition by the myriad of endocytic proteins with CBMs could potentially cause a block of endocytosis. Instead, the functional redundancy between the four interaction sites that we have described would allow endocytosis to proceed unhindered. Current models of CCV formation have attempted account for competition of a limited number of sites by proposing that the endocytic network must change over time (see discussion in 22). An expanded number of potential interaction sites on clathrin’s NTD would alleviate this bottleneck.
Materials and Methods
Antibodies and reagents
All laboratory chemicals were purchased from Sigma Aldrich or Fisher Scientific. Cell culture reagents were from Invitrogen. Alexa Fluor-546 and -647-conjugated human transferrin and goat anti-mouse Alexa Fluor-568 secondary antibody were from Molecular Probes. Transfection reagents Lipofectamine 2000 and GeneJuice were from Invitrogen and Novagen, respectively. Mouse monoclonal alpha-adaptin antibody (AP.6, clone ab2730) was from Abcam, anti-clathrin heavy chain antibody (TD.1) was purified from hybridoma CRL-2232, sheep anti-TGN46 (AHP500) was from AbD Serotec and anti-GFP was a kind gift from Prof. Francis Barr (University of Oxford, U.K.).
Molecular biology
The following plasmids were available from previous work: pBrain-GFP-CHC1 (Control), pBrain-GFP-CHC4 (GFP), pBrain-GFP-CHC(1-1675)KDP-CHC4 (CHC) and pBrain-GFP-CHC(331-1639)-CHC4 (ΔNTD) (32). The idiosyncratic nomenclature of these plasmids has three parts: i) pBrain denotes a plasmid with an H1 promoter for shRNA expression and a CMV promoter for protein expression; ii) the next part of the name denotes the protein that is expressed e.g. GFP-CHC(1-1675)KDP; iii) the last part indicate the shRNA that is expressed (CHC1 targets rat CHC and CHC4 targets human CHC)(31, 32). Yeast chc1p cDNA in pBG1805 was purchased from Open Biosystems. GFP-chc1p in pEGFP-C3 was made by inserting a XhoI-Acc65I PCR fragment into pEGFP-C3 to make GFP-chc1p(1-295), an Acc65I-BamHI fragment was ligated to give GFP-chc1p(1-1553), finally a BamHI-BamHI fragment was ligated to give full-length GFP-chc1p. When expressed in HEK293 cells, full-length GFP-chc1p was diffusely distributed and did not exhibit a typical clathrin distribution in pits and vesicles, even when co-expressed with mCherry-clc1p. Yeast chc1p with a human NTD (GFP-CHC(1-291)chc1p(298-1653)) was similarly ‘non-functional’ and so was not analysed further. The yeast-human chimera (YNTD, GFP-chc1p(1-297)CHC17(292-1675)) was made by inserting an Acc65IXmaI fragment from GFP-CHC(1-1675)KDP into GFP-chc1p. The remaining chimeric clathrin constructs and clathrin mutants were made by overlap extension PCR or site-directed mutagenesis. GFP-tagged clathrin constructs were converted to pBrain versions by ligating a BglII-EcoRV fragment into pBrain-GFP-CHC(1-1675)KDP-CHC4. For GFP-chc1p(1-100)CHC(101-1675), an Age I-EcoRV fragment was inserted into pBrain-GFP-CHC(1-1675)KDP-CHC4. For GFP-chc1p(1-297)CHC(292-1675), a BglII-Acc65I fragment was inserted into GFP-CHC(1-1675)KDP and then a BglII-EcoRV fragment was inserted into pBrain-GFP-CHC(1-1675)KDP-CHC4. Any constructs that contained the sequence coding for CHC17 residues 60-66 were knockdown-proof (31). Knock-down proof CHC has a serine at position 63, whereas wild type CHC has an arginine. All our CHC constructs used to date have S63 and we found no differences between S63 and R63 CHC constructs.
Sequence analysis
A multiple sequence alignment of CHC sequences [Homo sapiens NP_004850.1; Pan troglodytes XP_001135882.1; Canis familiaris XP_867227.1; Bos taurus NP_776448.1; Mus musculus NP_001003908.1; Rattus norvegicus NP_062172.1; Gallus gallus XP_415878.2; Danio rerio XP_699126.3, NP_001005391.1; Drosophila melanogaster NP_001096993.1; Anopheles gambiae XP_311856.3; Caenorhabditis elegans NP_499260.1; Schizosaccharomyces pombe NP_594148.1; Saccharomyces cerevisiae NP_011309.1; Kluyveromyces lactis XP_455531.1; Ashbya gossypii NP_985215.1; Magnaporthe oryzae XP_367864.1; Neurospora crassa XP_331709.1; Arabidopsis thaliana NP_187724.2, NP_187466.4; Oryza sativa NP_001065530.1] was done using CLUSTALW (http://www.ebi.ac.uk/tools). This alignment was used with the structure of the CHC NTD (PDB code 1UTC) to generate a model of sequence conservation on the NTD surface using CONSURF (http://consurf.tau.ac.il/). Conserved residues are shaded maroon and divergent residues are shaded cyan.
Cell culture and transfections
HEK293 and HeLa cells were maintained in DMEM supplemented with 10% fetal bovine serum and 100 units/ml penicillin/streptomycin at 37◻°C and 5% CO2. HEK293 cells were transfected by calcium phosphate method whereas HeLa cells were transfected using Lipofectamine 2000 or Gene Juice according to the manufacturers’ recommendations. HEK293 cells were analysed at three days post-transfection, when expression and CHC-depletion is maximal (31).
Knockdown of CHC in HeLa cells takes longer than in HEK293 cells (8, 47). We found that expression of pBrain plasmids over five days gave excellent clathrin-depletion but expression of GFP-CHCs was too low for rescue and detection of GFP was poor by flow cytometry. We therefore adopted a “two-hit” protocol where cells were transfected with siRNA on day 1, cells were then split and transferred to the new plates on day 2 and the transfection with appropriate pBrain plasmids was performed on day 3. Six hours after transfection, medium was replaced with fresh growth medium containing 5 mM sodium butyrate to increase efficiency of transfection and expression. After 16 hours (day 4), the media was replaced and cells were assayed on day 5. The siRNA was directed against human CHC (8) whereas for ‘Control’ cells, GL2 control siRNA (CGTACGCGGAATACTTCGA) was used followed by pBrain-GFP-CHC1 transfection. This protocol gave good depletion of endogenous clathrin as assessed by western blotting (Supplementary Fig. S3A).
Transferrin uptake and immunofluorescence
Transferrin uptake was assayed using microscopy and flow cytometry. For all transferrin uptake experiments, the cells were first incubated for 45 minutes at 37°C in serum-free DMEM. For microscopy, the cells were then incubated with 50 μg/mL Alexa Fluor 546–conjugated transferrin for 10 min at 37°C, washed, fixed, and mounted. For flow cytometry, a previous protocol was used (35). After serum deprivation, cells were trypsinized for 3 min at 37 °C, briefly pelleted and then incubated with 50 μg/ml Alexa Fluor 647–conjugated transferrin for 5 minutes at 4°C. The cells were moved to 37°C and incubated for 10 minutes. Similar results were obtained using 10 μg/ml transferrin. Cells were pelleted, washed once in PBS, acid-washed twice (0.1 M glycine, 150 mM NaCl, pH 3), and resuspended in PBS containing 1% BSA. The trypsinization does not interfere with transferrin binding and uptake (Supplementary Fig. S3B).
For immunofluorescence, cells on cover slips were fixed (3% PFA, 4% sucrose in PBS), permeabilized (PBS with 0.5 % Triton X-100) and blocked (PBS with 5% goat serum, 5% BSA) followed by incubations with primary then fluorescently-conjugated secondary antibodies in blocking solution with three five-minute washes following each incubation. Coverslips were mounted using Mowiol containing 4′,6-diamidino-2-phenylindole (DAPI).
Microscopy and flow cytometry
Confocal imaging was done using a Leica confocal microscope SP2 with a 63x (1.4 NA) oil-immersion objective. GFP and Alexa Fluor-546 or -568 were excited using an Ar/Kr 488 nm and the 543 line of a He/Ne laser, respectively. DAPI was excited using a multiphoton laser. Excitation and collection of emission were performed separately and sequentially at a depth of 8-bit. The initial transferrin uptake experiments in HEK293 cells were quantified exactly as described previously (30). In HeLa cells, microscopy was used as a qualitative measure only and all transferrin uptake was quantified by flow cytometry.
Flow cytometry was performed using a two-laser, four-color FACSCalibur flow cytometer (Becton Dickinson, Oxford, United Kingdom). Data analysis was done using CELLQuest (Becton Dickinson). Cells were gated so that only cells expressing GFP above a threshold, identical for all constructs, were included in the analysis. The median Alexa Fluor 647-transferrin signal was used to compare and plot data. Typically, 1000-10000 cells were analyzed per experiment. All experiments were repeated independently at least three times. Each time an experiment was run, the four controls were included (Control, GFP, CHC, ΔNTD). Control flow cytometry experiments show that uptake at 4 °C was significantly inhibited to ~10% of uptake at 37 °C (Supplementary Fig. 3B). In the GFP condition we saw 20-40% of uptake for CHC. The cells are gated for GFP only and so dead or dying cells could contribute to this signal. Alternatively, the difference could be due to residual clathrin in a small fraction of cells receiving only one hit of RNAi.
Note that trace levels of endogenous clathrin are unlikely to explain completely the assignment of mutants to either the functional or non-functional class. For this to be the case there would have to be higher levels of endogenous clathrin remaining in the sixteen ‘functional’ CHC mutants compared with the five ‘non-functional’ mutants; and this difference would have to be consistent over multiple experiments. The only variable between conditions is the amino acid sequence of the NTD, e.g. one amino acid change between the non-functional CDEG mutant versus the functional CDE mutant. For technical reasons, the amount of endogenous clathrin is difficult to measure following re-expression (Supplementary Fig S3A), but we found no strong evidence for differences in endogenous clathrin between functional and non-functional conditions.
For statistical tests, one-way ANOVA was used with Dunnet’s post-hoc test. Data analysis and presentation was done using Microscoft Excel, GraphPad Prism and Igor Pro 6.1 (Wavemetrics). Figures were assembled in Adobe Photoshop.
Supplementary Material
Figure S1
Figure S1: All clathrin constructs showed accumulation at the TGN. Representative confocal micrographs of a selection of the constructs used in this study to show the co-localization of TGN (middle, red in merge) and GFP-CHC constructs (left, green in merge) in cells depleted of endogenous clathrin. TGN accumulation can be seen for all constructs in Figs 1-5. Insets: 4X zoom of the boxed area Scale bar, 20 μm. This figure relates to Figures 1-5.
Figure S2
Figure S2: Assessment of the co-localization of clathrin constructs with AP-2 patches. A) Representative confocal micrographs to show the co-localization of AP-2 patches (middle, red in merge) and GFP-CHC constructs (left, green in merge) in cells depleted of endogenous clathrin. Insets: 4X zoom of the boxed area Scale bar, 20 μm. B) Bar charts to show the average intensity correlation quotient (ICQ) for GFP-CHCs and alpha-adaptin immunofluorescence. Bars show mean ± s.e.m. from four to eight cells from a single experiment. **, p <0.01. have been normalized to the ICQ value for CHC. Bars are colored to indicate functionality: green is functional, blue is non-functional. This figure relates to Figures 1-5.
Figure S3
Figure S3: Typical clathrin depletion and control flow cytometry data. A) Two examples of clathrin depletion as assessed by western blotting. HeLa cells prepared for flow cytometry experiments were lysed and separated by SDS-PAGE. Western blotting was carried out for clathrin (monoclonal anti-CHC, TD.1) and beta-tubulin as a loading control. An identical blot was probed with sheep anti-GFP. The blots show a reasonable depletion of clathrin that is not variable across different conditions. Note that HeLa cells are transfected first by siRNA and then by pBrain plasmids. Only a subset of cells (~20% for CHCs, ~40% for Control and GFP) are doubly transfected. In our flow cytometry experiments, cells are gated for GFP fluorescence meaning that only the double transfectants are analyzed. Here all cells are analyzed as the cells were not sorted by GFP fluorescence. Similarly the GFP blot is shown only to confirm the expression of CHC and NTD, which is too low for detection by TD.1. B) Typical profiles for transferrin uptake in control experiments. A negative population with no Alexa633-conjugated Transferrin is shown in blue. Uptake at 37 °C (red) or 4 °C (green) was measured with or without stripping of transferrin from the surface (dashed or solid line). Uptake at 4 °C was inhibited to ~10% of that at 37 °C. The cells were neither transfected nor gated. This figure relates to the Methods section.
Acknowledgements
We thank Linton Traub for encouragement and advice. Thanks also to Colin Rickman and members of the lab for useful discussion. AKW performed essentially all the experimental work. SJR conceived the project, carried out some early experiments and wrote the paper. This work was supported by a project grant from The Wellcome Trust (084569).
References
- 1.Brodsky FM, Chen CY, Knuehl C, Towler MC, Wakeham DE. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu Rev Cell Dev Biol. 2001;17:517–568. doi: 10.1146/annurev.cellbio.17.1.517. [DOI] [PubMed] [Google Scholar]
- 2.Traub LM. Tickets to ride: selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol. 2009;10(9):583–596. doi: 10.1038/nrm2751. [DOI] [PubMed] [Google Scholar]
- 3.Unanue ER, Ungewickell E, Branton D. The binding of clathrin triskelions to membranes from coated vesicles. Cell. 1981;26(3 Pt 1):439–446. doi: 10.1016/0092-8674(81)90213-0. [DOI] [PubMed] [Google Scholar]
- 4.Hirst J, Robinson MS. Clathrin and adaptors. Biochim Biophys Acta. 1998;1404(1-2):173–193. doi: 10.1016/s0167-4889(98)00056-1. [DOI] [PubMed] [Google Scholar]
- 5.Vigers GP, Crowther RA, Pearse BM. Location of the 100 kd-50 kd accessory proteins in clathrin coats. EMBO J. 1986;5(9):2079–2085. doi: 10.1002/j.1460-2075.1986.tb04469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vigers GP, Crowther RA, Pearse BM. Three-dimensional structure of clathrin cages in ice. EMBO J. 1986;5(3):529–534. doi: 10.1002/j.1460-2075.1986.tb04242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y, Boll W, Kirchhausen T, Harrison SC, Walz T. Cryo-electron tomography of clathrin-coated vesicles: structural implications for coat assembly. J Mol Biol. 2007;365(3):892–899. doi: 10.1016/j.jmb.2006.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Motley A, Bright NA, Seaman MN, Robinson MS. Clathrin-mediated endocytosis in AP-2-depleted cells. J Cell Biol. 2003;162(5):909–918. doi: 10.1083/jcb.200305145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reider A, Wendland B. Endocytic adaptors--social networking at the plasma membrane. J Cell Sci. 2011;124(Pt 10):1613–1622. doi: 10.1242/jcs.073395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ter Haar E, Musacchio A, Harrison SC, Kirchhausen T. Atomic structure of clathrin: a beta propeller terminal domain joins an alpha zigzag linker. Cell. 1998;95(4):563–573. doi: 10.1016/s0092-8674(00)81623-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fotin A, Cheng Y, Sliz P, Grigorieff N, Harrison SC, Kirchhausen T, Walz T. Molecular model for a complete clathrin lattice from electron cryomicroscopy. Nature. 2004;432(7017):573–579. doi: 10.1038/nature03079. [DOI] [PubMed] [Google Scholar]
- 12.ter Haar E, Harrison SC, Kirchhausen T. Peptide-in-groove interactions link target proteins to the beta-propeller of clathrin. Proc Natl Acad Sci USA. 2000;97(3):1096–1100. doi: 10.1073/pnas.97.3.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman OB, Jr., Krupnick JG, Gurevich VV, Benovic JL, Keen JH. Arrestin/clathrin interaction. Localization of the arrestin binding locus to the clathrin terminal domain. J Biol Chem. 1997;272(23):15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- 14.Drake MT, Traub LM. Interaction of two structurally distinct sequence types with the clathrin terminal domain beta-propeller. J Biol Chem. 2001;276(31):28700–28709. doi: 10.1074/jbc.M104226200. [DOI] [PubMed] [Google Scholar]
- 15.Miele AE, Watson PJ, Evans PR, Traub LM, Owen DJ. Two distinct interaction motifs in amphiphysin bind two independent sites on the clathrin terminal domain beta-propeller. Nat Struct Mol Biol. 2004;11(3):242–248. doi: 10.1038/nsmb736. [DOI] [PubMed] [Google Scholar]
- 16.Kang DS, Kern RC, Puthenveedu MA, von Zastrow M, Williams JC, Benovic JL. Structure of an arrestin2-clathrin complex reveals a novel clathrin binding domain that modulates receptor trafficking. J Biol Chem. 2009;284(43):29860–29872. doi: 10.1074/jbc.M109.023366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirchhausen T. Clathrin adaptors really adapt. Cell. 2002;109(4):413–416. doi: 10.1016/s0092-8674(02)00751-1. [DOI] [PubMed] [Google Scholar]
- 18.Dell’Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280(5362):431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- 19.Krupnick JG, Goodman OB, Jr., Keen JH, Benovic JL. Arrestin/clathrin interaction. Localization of the clathrin binding domain of nonvisual arrestins to the carboxy terminus. J Biol Chem. 1997;272(23):15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- 20.Dell’Angelica EC. Clathrin-binding proteins: got a motif? Join the network! Trends Cell Biol. 2001;11(8):315–318. doi: 10.1016/s0962-8924(01)02043-8. [DOI] [PubMed] [Google Scholar]
- 21.Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM. Molecular switches involving the AP-2 beta2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell. 2006;10(3):329–342. doi: 10.1016/j.devcel.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 22.Keyel PA, Thieman JR, Roth R, Erkan E, Everett ET, Watkins SC, Heuser JE, Traub LM. The AP-2 adaptor beta2 appendage scaffolds alternate cargo endocytosis. Mol Biol Cell. 2008;19(12):5309–5326. doi: 10.1091/mbc.E08-07-0712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundmark R, Carlsson SR. The beta-appendages of the four adaptor-protein (AP) complexes: structure and binding properties, and identification of sorting nexin 9 as an accessory protein to AP-2. Biochem J. 2002;362(Pt 3):597–607. doi: 10.1042/0264-6021:3620597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Owen DJ, Vallis Y, Pearse BM, McMahon HT, Evans PR. The structure and function of the beta 2-adaptin appendage domain. EMBO J. 2000;19(16):4216–4227. doi: 10.1093/emboj/19.16.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knuehl C, Chen CY, Manalo V, Hwang PK, Ota N, Brodsky FM. Novel binding sites on clathrin and adaptors regulate distinct aspects of coat assembly. Traffic. 2006;7(12):1688–1700. doi: 10.1111/j.1600-0854.2006.00499.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmid EM, McMahon HT. Integrating molecular and network biology to decode endocytosis. Nature. 2007;448(7156):883–888. doi: 10.1038/nature06031. [DOI] [PubMed] [Google Scholar]
- 27.Collette JR, Chi RJ, Boettner DR, Fernandez-Golbano IM, Plemel R, Merz AJ, Geli MI, Traub LM, Lemmon SK. Clathrin functions in the absence of the terminal domain binding site for adaptor-associated clathrin-box motifs. Mol Biol Cell. 2009;20(14):3401–3413. doi: 10.1091/mbc.E08-10-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baggett JJ, Wendland B. Clathrin function in yeast endocytosis. Traffic. 2001;2(5):297–302. doi: 10.1034/j.1600-0854.2001.002005297.x. [DOI] [PubMed] [Google Scholar]
- 29.Yeung BG, Phan HL, Payne GS. Adaptor complex-independent clathrin function in yeast. Mol Biol Cell. 1999;10(11):3643–3659. doi: 10.1091/mbc.10.11.3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hood FE, Royle SJ. Functional equivalence of the clathrin heavy chains CHC17 and CHC22 in endocytosis and mitosis. J Cell Sci. 2009;122(Pt 13):2185–2190. doi: 10.1242/jcs.046177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royle SJ, Bright NA, Lagnado L. Clathrin is required for the function of the mitotic spindle. Nature. 2005;434(7037):1152–1157. doi: 10.1038/nature03502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Royle SJ, Lagnado L. Trimerisation is important for the function of clathrin at the mitotic spindle. J Cell Sci. 2006;119(Pt 19):4071–4078. doi: 10.1242/jcs.03192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Borner GH, Harbour M, Hester S, Lilley KS, Robinson MS. Comparative proteomics of clathrin-coated vesicles. J Cell Biol. 2006;175(4):571–578. doi: 10.1083/jcb.200607164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nesterov A, Carter RE, Sorkina T, Gill GN, Sorkin A. Inhibition of the receptor-binding function of clathrin adaptor protein AP-2 by dominant-negative mutant mu2 subunit and its effects on endocytosis. EMBO J. 1999;18(9):2489–2499. doi: 10.1093/emboj/18.9.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Motley AM, Berg N, Taylor MJ, Sahlender DA, Hirst J, Owen DJ, Robinson MS. Functional Analysis of AP-2 {alpha} and {micro}2 Subunits. Mol Biol Cell. 2006;17(12):5298–5308. doi: 10.1091/mbc.E06-05-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Glaser F, Pupko T, Paz I, Bell RE, Bechor-Shental D, Martz E, Ben-Tal N. ConSurf: identification of functional regions in proteins by surface-mapping of phylogenetic information. Bioinformatics. 2003;19(1):163–164. doi: 10.1093/bioinformatics/19.1.163. [DOI] [PubMed] [Google Scholar]
- 37.Brodsky FM. Living with clathrin: its role in intracellular membrane traffic. Science. 1988;242(4884):1396–1402. doi: 10.1126/science.2904698. [DOI] [PubMed] [Google Scholar]
- 38.Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization. J Neurosci. 2004;24(16):4070–4081. doi: 10.1523/JNEUROSCI.0346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor MJ, Perrais D, Merrifield CJ. A high precision survey of the molecular dynamics of mammalian clathrin-mediated endocytosis. PLoS biology. 2011;9(3):e1000604. doi: 10.1371/journal.pbio.1000604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu SH, Marks MS, Brodsky FM. A dominant-negative clathrin mutant differentially affects trafficking of molecules with distinct sorting motifs in the class II major histocompatibility complex (MHC) pathway. J Cell Biol. 1998;140(5):1023–1037. doi: 10.1083/jcb.140.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilde A, Beattie EC, Lem L, Riethof DA, Liu SH, Mobley WC, Soriano P, Brodsky FM. EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell. 1999;96(5):677–687. doi: 10.1016/s0092-8674(00)80578-4. [DOI] [PubMed] [Google Scholar]
- 42.Moskowitz HS, Yokoyama CT, Ryan TA. Highly cooperative control of endocytosis by clathrin. Mol Biol Cell. 2005;16(4):1769–1776. doi: 10.1091/mbc.E04-08-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Doyon JB, Zeitler B, Cheng J, Cheng AT, Cherone JM, Santiago Y, Lee AH, Vo TD, Doyon Y, Miller JC, Paschon DE, Zhang L, Rebar EJ, Gregory PD, Urnov FD, et al. Rapid and efficient clathrin-mediated endocytosis revealed in genome-edited mammalian cells. Nat Cell Biol. 2011;13(3):331–337. doi: 10.1038/ncb2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Kleist L, Stahlschmidt W, Bulut H, Gromova K, Puchkov D, Robertson MJ, Macgregor KA, Tomlin N, Pechstein A, Chau N, Chircop M, Sakoff J, von Kries JP, Saenger W, Krausslich HG, et al. Role of the clathrin terminal domain in regulating coated pit dynamics revealed by small molecule inhibition. Cell. 2011;146(3):471–484. doi: 10.1016/j.cell.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Zhuo Y, Ilangovan U, Schirf V, Demeler B, Sousa R, Hinck AP, Lafer EM. Dynamic interactions between clathrin and locally structured elements in a disordered protein mediate clathrin lattice assembly. J Mol Biol. 2010;404(2):274–290. doi: 10.1016/j.jmb.2010.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morgan JR, Prasad K, Hao W, Augustine GJ, Lafer EM. A conserved clathrin assembly motif essential for synaptic vesicle endocytosis. J Neurosci. 2000;20(23):8667–8676. doi: 10.1523/JNEUROSCI.20-23-08667.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Booth DG, Hood FE, Prior IA, Royle SJ. A TACC3/ch-TOG/clathrin complex stabilises kinetochore fibres by inter-microtubule bridging. EMBO J. 2011;30(5):906–919. doi: 10.1038/emboj.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1
Figure S1: All clathrin constructs showed accumulation at the TGN. Representative confocal micrographs of a selection of the constructs used in this study to show the co-localization of TGN (middle, red in merge) and GFP-CHC constructs (left, green in merge) in cells depleted of endogenous clathrin. TGN accumulation can be seen for all constructs in Figs 1-5. Insets: 4X zoom of the boxed area Scale bar, 20 μm. This figure relates to Figures 1-5.
Figure S2
Figure S2: Assessment of the co-localization of clathrin constructs with AP-2 patches. A) Representative confocal micrographs to show the co-localization of AP-2 patches (middle, red in merge) and GFP-CHC constructs (left, green in merge) in cells depleted of endogenous clathrin. Insets: 4X zoom of the boxed area Scale bar, 20 μm. B) Bar charts to show the average intensity correlation quotient (ICQ) for GFP-CHCs and alpha-adaptin immunofluorescence. Bars show mean ± s.e.m. from four to eight cells from a single experiment. **, p <0.01. have been normalized to the ICQ value for CHC. Bars are colored to indicate functionality: green is functional, blue is non-functional. This figure relates to Figures 1-5.
Figure S3
Figure S3: Typical clathrin depletion and control flow cytometry data. A) Two examples of clathrin depletion as assessed by western blotting. HeLa cells prepared for flow cytometry experiments were lysed and separated by SDS-PAGE. Western blotting was carried out for clathrin (monoclonal anti-CHC, TD.1) and beta-tubulin as a loading control. An identical blot was probed with sheep anti-GFP. The blots show a reasonable depletion of clathrin that is not variable across different conditions. Note that HeLa cells are transfected first by siRNA and then by pBrain plasmids. Only a subset of cells (~20% for CHCs, ~40% for Control and GFP) are doubly transfected. In our flow cytometry experiments, cells are gated for GFP fluorescence meaning that only the double transfectants are analyzed. Here all cells are analyzed as the cells were not sorted by GFP fluorescence. Similarly the GFP blot is shown only to confirm the expression of CHC and NTD, which is too low for detection by TD.1. B) Typical profiles for transferrin uptake in control experiments. A negative population with no Alexa633-conjugated Transferrin is shown in blue. Uptake at 37 °C (red) or 4 °C (green) was measured with or without stripping of transferrin from the surface (dashed or solid line). Uptake at 4 °C was inhibited to ~10% of that at 37 °C. The cells were neither transfected nor gated. This figure relates to the Methods section.