Decreased insulin sensitivity and increased oxidative damage in wasting adipose tissue depots of wild-type mice (original) (raw)
Abstract
Unintentional weight loss (wasting) in the elderly is a major health concern as it leads to increased mortality. Several studies have focused on muscle loss, but little is known about the mechanisms giving rise to loss of fat mass at old ages. To investigate potential mechanisms, white adipose tissue (WAT) characteristics and proteomic profiles were compared between adult (10–12-month-old) and aged (22–24-month-old) wild-type mice. Four individual WAT depots were analyzed to account for possible depot-specific differences. Proteomic profiles of WAT depots, along with body weights and compositions, plasma levels of insulin, leptin and adiponectin, insulin tolerance, adipocyte sizes, and products of oxidative damage in each WAT depot were determined. We found that lean mass remained constant while fat mass and insulin tolerance were decreased in old age, as were adipocyte sizes in the WAT depots. Proteomic results showed increased levels of enolase, pyruvate dehydrogenase E1β, NAD+−dependent isocitrate dehydrogenase α, and ATP synthase subunit β, and decreased levels of carbonic anhydrase 3 in WAT of aged mice. These data suggest increased aerobic glucose oxidation in wasting WAT, consistent with decreased insulin signaling. Also, Cu/Zn superoxide dismutase and two chaperones were increased in aged WAT depots, indicating higher stress resistance. In agreement, lipid peroxidation (HNE-His adducts) increased in old age, although protein oxidation (carbonyl groups) showed no increase. In conclusion, features of wasting WAT were similar in the four depots, including decreased adipocyte sizes and alterations in protein expression profiles that indicated decreased insulin sensitivity and increased lipid peroxidation.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-011-9304-7) contains supplementary material, which is available to authorized users.
Keywords: Wasting, Aging, White adipose tissue depots, Proteomics, Oxidative damage, Stress resistance, Insulin resistance
Introduction
Very old age (>85 years) is generally characterized by unintentional weight loss that usually predicts all-cause mortality (Dorner and Rieder 2011; Miller and Wolfe 2008). This “wasting” phenomenon includes loss of muscle mass (sarcopenia) and fat mass resulting in generalized frailty (Silver et al. 1993; Miller and Wolfe 2008). Unintentional weight loss is a major health concern in the elderly population because of its detrimental effects on functionality and independence. Several reviews have focused on its possible causes and plausible therapeutic approaches (Clegg and Young 2011; Visvanathan and Chapman 2009; Thorner 2009; Hickson 2006; Roberts and Rosenberg 2006). To date, the mechanisms leading to age-related sarcopenia have been widely investigated, but there is less information about the processes resulting in decreased fat mass in the elderly.
The mechanisms of fat loss have been investigated more in depth in cachexia, which is another condition that involves wasting. Cachexia is usually associated with underlying disease, such as cancer, chronic kidney disease, congestive heart failure, etc. (Hickson 2006). Available data suggest that the loss of fat mass in cancer cachexia precedes the loss of muscle (Fouladiun et al. 2005). Interestingly, trunk fat appears to decrease at a faster rate than fat in the limbs as cachexia progresses, suggesting depot-specific effects of WAT wasting (Fouladiun et al. 2005). Studies have reported increased lipolysis, low lipoprotein lipase (LPL) activity, and low adipogenic signals as the underlying mechanisms that result in fat loss in cancer cachexia (Das et al. 2011; Bing and Trayhurn 2008; Agustsson et al. 2007). Consistently, adipocyte volumes decrease, while their number is conserved (Bing and Trayhurn 2008; Dahlman et al. 2010). Even though age-related wasting is usually indicative of underlying disease (Miller and Wolfe 2008), it is not clear if similar mechanisms as cachexia may induce wasting in the elderly.
We have previously reported that male C57BL/6J mice lose weight at old age (19–24 months), and that the main component of this loss is represented by a decrease in fat mass (Berryman et al. 2010). Characterizing the underlying mechanisms that lead to the loss of fat mass in aged mice may help understand unintentional fat loss in elderly humans. In a previous report, we characterized white adipose tissue (WAT) depots of adult (12 months old) C57BL/6J mice and reported several WAT depot-specific differences (Sackmann-Sala et al. 2011). In the current study, we analyzed WAT depots of aged (24 months old) mice and compared them to our previous data. Four WAT depots (inguinal, retroperitoneal, mesenteric, and epididymal) were investigated, given the current appreciation of depot-specific differences and the reported influence of location on fat mass loss in humans (Sackmann-Sala et al. 2011; Berryman et al. 2011; Cartwright et al. 2010; Tran et al. 2008; Wajchenberg et al. 2002; Kirkland et al. 1990). Proteomic profiles of the WAT depots, along with body weight and composition, plasma levels of insulin, leptin, total and high molecular weight (HMW) adiponectin, insulin tolerance, adipocyte sizes and products of oxidative damage were measured. Our results showed that, while WAT depots are heterogeneous in nature, the effects of old age on WAT characteristics were similar among depots. The observed features of wasting WAT are consistent with decreased insulin sensitivity and increased reactive oxygen species (ROS) production in WAT depots of aged mice.
Methods
Mice
Male wild-type C57BL/6J mice (adult: 10–12; aged: 22–24 months old) were used in this study. WAT depot-specific proteomes, depot weights and protein contents, and adipocyte sizes of the adult group had been analyzed previously (Sackmann-Sala et al. 2011). All mice were kept under specific pathogen-free conditions on a 14/10-hour light/dark cycle, with normal chow (Prolab RMH 3000 LabDiet; PMI Richmond, Richmond, IN) and water provided ad libitum. Procedures were approved by the Ohio University Animal Care and Utilization Committee.
Body composition
Body composition was measured on live mice using a quantitative NMR apparatus (Minispec, Bruker Optics, Billerica, MA).
Plasma insulin, leptin and adiponectin and insulin tolerance tests
Mice were fasted for eight to 12 h and blood from the tail vein was collected in heparinized tubes. Plasma was then separated by centrifugation at 7,000 g for 10 min and stored at −80°C until processing. Insulin levels were measured using an ELISA kit from ALPCO Diagnostics, Salem, NH (80-INSMSU-E01). Leptin levels were quantified using an ELISA kit from R&D Systems, Inc., Minneapolis, MN (MOB00) or Crystal Chem Inc., Downers Grove, IL (90030). HMW and total adiponectin levels were measured using an ELISA kit from ALPCO Diagnostics (47-ADPMS-E01). Insulin tolerance tests (ITT) were performed on nonfasted mice using human insulin (Humulin-R, Ely Lilly, Indianapolis, IN) at 0.75 U/kg body weight. Mice were injected intraperitoneally and glucose was measured every 15 min for 1 h after injection.
WAT depot samples
Mice were sacrificed by cervical dislocation and inguinal, retroperitoneal, mesenteric and epididymal WAT were collected and weighed. Samples for proteomics (n = 6 mice per age group) were snap frozen in liquid nitrogen and stored at −80°C until processing; samples for histology (n = 6 mice per age group) were fixed in 10% phosphate buffered formalin.
Proteomic analysis
The procedures for 2-dimensional gel electrophoresis (2DE) were based on those described previously (Cruz-Topete et al. 2011a; Cruz-Topete et al. 2011b; Cruz-Topete et al. 2011c; Christensen et al. 2011; Ding et al. 2011; Ding and Kopchick 2011; Okada et al. 2010; Sackmann-Sala et al. 2009; List et al. 2007; Qiu et al. 2005) and have been described in detail by Sackmann-Sala et al. (2011). For sample homogenization, WAT samples were thawed and diluted in sample buffer (7 M urea, 2 M thiourea, 1% w/v sulfobetaine-10, 3% w/v CHAPS, 0.25% v/v Bio-Lyte 3/10 ampholytes (Bio-Rad Laboratories, Inc., Hercules, CA), and 1.5% v/v protease inhibitor cocktail (Sigma, St. Louis, MO)). Mechanical homogenization was followed by brief sonication, incubation at 35°C for 15 min, and centrifugation at 16,000 g for 45 min at room temperature. Protein solutions were transferred to clean tubes after floating lipid layers were removed. Protein concentration in each WAT sample was measured using Bio-Rad Protein Assay. Protein content per gram of tissue was estimated based on the volume of homogenate and the initial weight of the sample. Details of the 2DE protocol used are included as Online Resource 1.
Mass Spectrometry (MS)
Protein spots displaying significant intensity changes between age groups and among WAT depots were manually excised from the gels and sent to Protea Biosciences Inc., Morgantown, WV for analysis by MS and tandem-MS (MS/MS) using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) and MALDI-TOF–TOF, respectively. These procedures have been described in detail in Sackmann-Sala et al. (2011) and are included as Online Resource 1. Data processing was performed using the software Applied Biosystems GPS Explorer v3.6 or ProteinPilot 3.0.
Protein identification (performed at Ohio University)
Protein identities obtained by Protea Biosciences were verified or revised using the MS and MS/MS data obtained and the online software Mascot (www.matrixscience.com). Search parameters have been described previously (Sackmann-Sala et al. 2011) and are included as Online Resource 1.
Oxidative stress products
Protein carbonyls were measured as an indication of protein oxidation in WAT samples using an OxiSelect Protein Carbonyl ELISA kit (Cell Biolabs, Inc., San Diego, CA). Samples were homogenized as described for proteomics. Protein carbonyl content was determined by comparison to a standard curve prepared with reduced and oxidized BSA standards. HNE-His protein adducts, indicative of lipid peroxidation, were detected using an OxiSelect HNE-His Adduct ELISA kit (Cell Biolabs). HNE-protein adducts were quantified by comparison to a standard curve generated with predetermined HNE-BSA standards.
Histology and adipocyte sizing
Five-micrometer sections of paraffin-embedded WAT samples were stained with hematoxylin and eosin. Slides were examined using a Nikon Eclipse E600 microscope under 200 × −400× magnification, and images from three non-overlapping fields were obtained with a SPOT RT digital camera. The mean adipocyte size (cross-sectional area) calculated for each WAT sample included all the adipocytes present in the three field images. Measurements were performed as described by Chen and Farese (2002). For the estimation of adipocyte numbers, the cross-sectional area of each adipocyte was converted to volume [4/3 × Π × (area / Π)3/2], and its mass was calculated based on the density of triolein (0.915 g/ml). Then, each WAT depot weight was divided by the mean adipocyte mass in that location to estimate the number of cells (Tchoukalova et al. 2010).
Statistical analysis
Data for body weight, body composition, plasma hormone levels and adipocyte size and number in each WAT depot were compared between age groups using a two-tailed independent samples _t_-test. The homogeneity of variances between groups was tested with Levene’s test and when homoscedasticity was not met (P < 0.05), _t_-test results were adjusted.
Spot intensity data were log-transformed. WAT depot weights, protein contents, protein carbonyls, HNE-His adducts, and log-transformed spot intensities were compared between age groups and among depots using a two-way ANOVA with one within-subjects factor (depot) and one between-subjects factor (age). Sphericity was tested using Mauchly’s method, and a Greenhouse–Geisser correction was applied when the assumption of sphericity was not met (P < 0.05). When significant differences were found between age groups and/or among depots, Tukey’s HSD post hoc tests were performed. The same tests were applied for the analysis of the effects of age on ITTs. Correlations were evaluated using the Pearson test.
Statistical significance cutoffs were P < 0.01 for spot intensity differences between age groups and among depots (given the intrinsic variability of the 2DE technique) and P < 0.05 for all other comparisons and for correlations. The softwares used were SPSS v14.0 and SigmaPlot v11; post hoc tests for nonspherical data were performed manually in Excel spreadsheets.
Results
Body weight, body composition, plasma hormone levels, and insulin tolerance
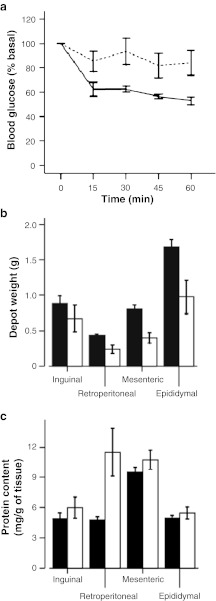
Mean values for body weight, body composition and plasma levels of insulin, leptin, total and HMW adiponectin for the adult and aged groups are shown in Table 1. Although body weight and lean mass remained very similar between groups, fat mass was significantly decreased in aged compared to adult mice, resulting in significant differences in percent fat and percent lean mass between age groups. Because lean mass includes more than skeletal muscle, the weights of liver, heart and kidney were recorded at dissection and compared between groups. No significant changes were detected in the weights of these organs between adult and aged animals (data not shown). Plasma insulin and adiponectin (HMW and total) levels were significantly lower in the aged group, and a similar trend was observed for leptin levels, although the difference did not reach significance. Moreover, ITTs showed that mice undergoing WAT wasting had a significantly decreased response to insulin (Fig. 1a).
Table 1.
Body weight, body composition and hormone levels of adult and aged mouse groups
| Adult (n = 10–18) | Aged (n = 13–23) | _P_-value | |
|---|---|---|---|
| Age (months) | 10–12 | 22–24 | |
| Body weight (g) | 36.5 ± 1.0 | 37.8 ± 1.4 | 0.495 |
| Body composition | |||
| Fat mass (g) | 8.4 ± 0.7 | 5.5 ± 1.1 | 0.033* |
| Percent fat mass | 21.8 ± 1.6 | 13.8 ± 2.0 | 0.005* |
| Lean mass (g) | 24.9 ± 0.6 | 26.3 ± 0.6 | 0.099 |
| Percent lean mass | 67.0 ± 1.2 | 72.6 ± 1.8 | 0.014* |
| Insulin (ng/ml) | 3.16 ± 0.58 | 1.53 ± 0.29 | 0.026* |
| Leptin (ng/ml) | 31.4 ± 5.1 | 14.4 ± 6.7 | 0.071 |
| Adiponectin | |||
| Total (μg/ml) | 29.1 ± 2.0 | 20.4 ± 1.7 | 0.003* |
| HMW (μg/ml) | 10.6 ± 1.4 | 6.2 ± 0.8 | 0.015* |
| HMW/total | 0.35 ± 0.03 | 0.29 ± 0.02 | 0.049* |
| Total adiponectin/leptin | 1.1 ± 0.1 | 7.1 ± 2.8 | 0.055 |
Fig. 1.
Insulin tolerance tests (ITTs) and depot-specific weight and protein content of adult and aged mice. Data shown are the mean±SE. a Insulin tolerance was tested on 10-month-old mice (solid line, n = 6) and 22-month-old mice (dotted line, n = 10) and significant effects of age (P = 0.049) were found. b WAT depot weight means for 12-month-old mice (black bars, n = 12) and 24-month-old mice (white bars, n = 14). A two-way ANOVA showed a significant effect of age (P = 0.019), depot (P < 0.001) and age × depot interaction (P = 0.020) for depot weight. c Protein content in WAT depots of 12-month-old mice (black bars, n = 6) and 24-month-old mice (white bars, n = 6). A two-way ANOVA showed a significant effect of depot (P < 0.001) and significant age × depot interaction (P = 0.011) with no effect of age
Depot-specific weight and protein content
When depot weights for both 12- and 24-month-old mice were analyzed, there was a significant decrease in all depot weights with age (P = 0.019) (Fig. 1b). Among WAT depots, mean weight was significantly higher in the epididymal depot than the others, and the inguinal depot was significantly larger than the retroperitoneal one. The decrease with age was more marked in the epididymal depot, which in aged mice displayed a similar weight to the inguinal depot. Regarding protein content per gram of tissue, there were trends to increase in wasting WAT depots, but this increase was only significant in the retroperitoneal depot (P = 0.011 for age × depot interaction). Also, mesenteric and retroperitoneal WAT showed higher levels of protein content than inguinal and epididymal depots (Fig. 1c).
Correlations of hormone levels to total and depot-specific fat mass
We evaluated correlations between the levels of insulin, leptin, total and HMW adiponectin with total and depot-specific fat masses. Leptin levels in adult and aged mice correlated positively with total fat mass (_r_2 = 0.77, n = 10, P = 0.001; _r_2 = 0.93, n = 10, P < 0.001, respectively), as shown in Online Resource 2. Interestingly, insulin, total and HMW adiponectin levels correlated positively with fat mass in the aged group only (insulin: _r_2 = 0.58, n = 10, P = 0.011; total adiponectin: _r_2 = 0.54, n = 10, P = 0.015; HMW adiponectin: _r_2 = 0.51, n = 10, P = 0.020) (Online Resource 2). Similarly, only in aged mice, the weights of the individual WAT depots correlated significantly and positively with the levels of insulin and leptin (Online Resource 3). In contrast, in adult mice, the only significant correlations found were between the weights of the mesenteric and inguinal WAT depots and circulating leptin levels (Online Resource 3). Correlations between insulin and leptin were also significant in aged mice only (Online Resource 3).
Adipocyte size and number
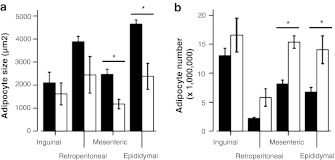
Mean adipocyte sizes in WAT depots of the adult group have been reported (Sackmann-Sala et al. 2011) and were significantly higher in epididymal WAT than inguinal and mesenteric depots, with retroperitoneal WAT showing intermediate values. When both age groups were compared, a decrease in adipocyte size with wasting was observed in all WAT depots, although this decrease was only significant in mesenteric and epididymal WAT (Fig. 2a). Adipocyte numbers increased reciprocally with wasting, reaching significance in mesenteric and epididymal depots (Fig. 2b).
Fig. 2.
Age effects in adipocyte size a and number b in the WAT depots. Data shown are the mean±SE and include results obtained from six mice in each age group (except retroperitoneal WAT in the aged group, which could only be analyzed in three mice). Significant differences (P < 0.05) between age groups in each WAT depot (independent _t_-test) are shown with an asterisk. (black bars) 12-month-old mice; (white bars) 24-month-old mice
Effects of age on WAT proteomes
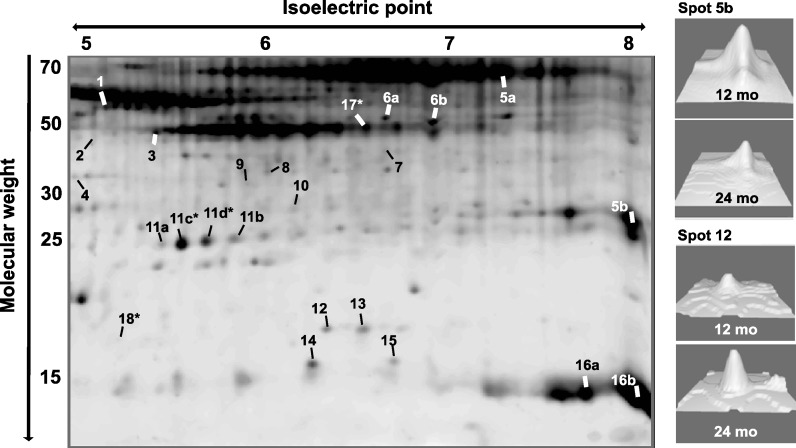
WAT samples from four WAT depots of 24-month-old mice were resolved by 2DE and compared to the samples from 12-month-old mice analyzed previously (Sackmann-Sala et al. 2011). The intensities of 169 spots that could be distinguished clearly and matched among all gels were evaluated. Twenty spots showed significant effects of age (wasting) and four displayed significant interactions of age × depot (Fig. 3 and Online Resources 4 to 6).
Fig. 3.
Representative 2D-gel of WAT showing spots that displayed significant intensity differences (P < 0.01) between age groups (20 spots) or significant interaction of age × depot (four spots, marked with an asterisk). Numbered labels correspond to protein identities shown in Table 2. The right panel shows 3D views of intensity peaks for spots 5b and 12, as examples of age-related changes in WAT. Images were obtained using the 3D viewer tool from PDQuest, which converts spot intensity data to topographical peaks and valleys
Using MS and MS/MS, 18 unique proteins were identified that displayed significant age-dependent intensity changes, with some spots representing slightly modified isoforms of the same protein (Online Resource 7). These isoforms probably result from posttranslational modifications, which generate subtle changes in the p_I_ and/or molecular weight of the proteins. Two spots (2 and 7) could not be identified, probably because of the low protein amount present in these spots. Table 2 summarizes the proteins identified, how they were affected by wasting, and their biological functions (generation of ATP, glucose and lipid metabolism, lipid transport, stress resistance, cytoskeleton structure, etc.).
Table 2.
Summary of protein identities, behavior with age, and protein function for spots displaying significant intensity differences between 12- and 24-month-old mice (P < 0.01)
| Spot | Protein identity | Difference | Function |
|---|---|---|---|
| ATP generation | |||
| 1a | ATP synthase subunit β, mitochondrial | 12 mo < 24 mo | ATP synthesis |
| Glucose/lipid metabolism | |||
| 5a–ba | Carbonic anhydrase 3 | 12 mo > 24 mo | pH regulation; provide HCO−3 for carbon-fixing reactions |
| 6a–b | Enolase | 12 mo < 24 mo | Glycolysis/glyceroneogenesis |
| 8 | Pyruvate dehydrogenase E1 β, mitochondrial | 12 mo < 24 mo | Conversion of pyruvate to acetyl CoA |
| 9 | Isocitrate dehydrogenase [NAD+] α | 12 mo < 24 mo | Krebs cycle |
| 15 | Fatty acid-binding protein, epidermal | 12 mo < 24 mo | Lipolysis |
| Stress resistance | |||
| 12 | Superoxide dismutase [Cu–Zn] | 12 mo < 24 mo | Antioxidant protein |
| 10 | Endoplasmic reticulum resident protein 29 | 12 mo < 24 mo | Chaperone |
| 13 | Peptidyl-prolyl cis-trans isomerase A | 12 mo < 24 mo | Chaperone |
| Lipid transport | |||
| 3a | Apolipoprotein A-IV | 12 mo < 24 mo | Cholesterol transport in HDL and chylomicrons, anti-inflammatory functions |
| 11a–d | Apolipoprotein A-I | a–b: 12 mo < 24 mo | Cholesterol transport in HDL, anti-inflammatory functions |
| c–d: Ing, Epi 12 mo < 24 mo | |||
| d: Epi < Ret at 12 mo | |||
| Cytoskeleton | |||
| 1a | Vimentin | 12 mo < 24 mo | Class III intermediate filaments |
| 3a | Actin | 12 mo < 24 mo | Microfilaments; muscle contraction |
| 17 | Actin | Ret < Mes at 12 mo | Microfilaments; muscle contraction |
| Other | |||
| 4 | Annexin A5 | 12 mo > 24 mo | Anticoagulant protein |
| 5aa | Serum albumin | 12 mo > 24 mo | Most abundant plasma protein, transporter, regulator of colloidal osmotic pressure in blood |
| 14 | Transthyretin | 12 mo < 24 mo | Binds thyroid hormones and RBP4 |
| 16a–b | Hemoglobin subunit β-1 | 12 mo > 24 mo | Oxygen transport |
| 18 | EH domain-containing protein 2 | Ing > Epi at 24 mo | Regulation of endocytosis |
Products of oxidative damage
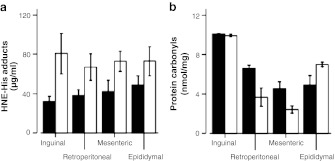
Lipid peroxidation and protein oxidation were measured in the WAT depots as 4-hydroxynonenal (HNE)-His adducts and protein carbonyl content, respectively (Halliwell and Whiteman 2004). HNE-protein adducts were significantly higher in wasting WAT depots (P = 0.021) when compared to adult tissue, with no depot effect or age × depot interaction (Fig. 4a). In contrast, protein carbonyl content was not different between age groups but showed significant effects of depot (P < 0.001) and significant interaction of age × depot (P < 0.001). Post hoc tests showed protein carbonyl levels to be significantly higher in inguinal WAT than in the other depots, and the retroperitoneal and epididymal depots to be higher than mesenteric WAT. Also, retroperitoneal WAT showed a significant decrease in protein carbonyl content in aged compared to adult animals (Fig. 4b).
Fig. 4.
Oxidative damage products measured in WAT depots of 12- and 24-month-old mice (black and white bars, respectively). Data shown are the mean±SE for five male mice. a HNE-protein adducts were significantly different between age groups (P = 0.021), with no effect of depot or age × depot interaction. b Protein carbonyl content showed no differences between age groups, except for a decrease with age in the retroperitoneal WAT depot (age × depot interaction P < 0.001). On the other hand, differences among depots showed the highest levels in inguinal WAT, followed by retroperitoneal and epididymal WAT, and lowest in the mesenteric depot (P < 0.001)
Discussion
The goal of this study was to analyze the characteristics and proteomic profiles of WAT depots from aged mice, in order to identify possible mechanisms of age-related fat loss. Healthy adult mice (10–12 months old) were compared to old mice (22–24 months old). The 22–24-month-old group represents a stage of healthy aging in mice, given that after 24 months of age, more than 50% of ad libitum fed C57BL/6 mice develop cancer (Ikeno et al. 2009). To avoid the presence of this confounding factor, we did not include mice older than 24 months in our study. Based on longitudinal data in this strain of mice (Berryman et al. 2010), the two age groups studied represent a period of fat accumulation in adulthood (10–12-month-old mice) and a period of fat loss at older age (22–24-month-old mice). Despite the old age of mice in the 22–24-month-old group, only fat mass (and not lean mass) decreased in that group, consistent with previous studies (Berryman et al. 2010; Bonkowski et al. 2006). Others have reported decreases in skeletal muscle mass in C57Bl/6 J mice, but at later ages (28–32 months) (Muller et al. 2007b). Therefore, the loss of fat mass seems to take place at an earlier time point than the loss of muscle mass in aging mice. The importance of fat mass in maintaining functionality in the elderly population often is not recognized (Morley 2007), and it is the decrease in lean mass that has received most of the attention (Clegg and Young 2011; Visvanathan and Chapman 2009; Miller and Wolfe 2008; Hickson 2006; Roberts and Rosenberg 2006). However, paradoxically beneficial effects of obesity in elderly subjects are now starting to be appreciated (Dorner and Rieder 2011). These findings are still controversial and might be affected by selection bias, as suggested by a recent report (Singh et al. 2011). In any case, the existence of a cause–effect relationship between fat mass loss and health span in advanced age is a topic of current debate. More research is needed to establish whether fat loss may initiate the causes of disease and death or if underlying disease, leading to death, may trigger a decrease in fat mass.
Together with the decreased fat mass, circulating levels of insulin, leptin, and total and HMW adiponectin were all decreased in aged/wasting mice, although the differences in leptin did not reach significance. In agreement, lower insulin and leptin levels have been reported for weight loss in humans (Agustsson et al. 2007). Although insulin secretion was not measured in this study, decreased insulin secretion in old age could account for the lower insulin tolerance observed in the wasting group. Decreased adiponectin action in aged mice was consistent with their decreased response to insulin in an ITT. Overall, these data reflect the insulin resistant state generally associated with old age (Narimiya et al. 1984; DeFronzo 1981). Interestingly, the [HMW/total adiponectin] ratio was significantly lower in aged compared to adult mice, supporting a role for HMW adiponectin as the insulin-sensitizing form of this hormone (Kaser et al. 2008). However, the ratio of [total adiponectin/leptin], another suggested indicator of insulin sensitivity (Lee et al. 2009) tended to increase in aged mice compared to the adult group. Therefore, this ratio might not be a good indicator of insulin sensitivity in older/wasting mice. Of note is that total adiponectin levels appear to increase and not decrease in elderly humans (Kizer et al. 2010).
Correlations were tested between fat mass and the four plasma hormone levels measured. Strong positive correlations between fat mass and plasma levels of insulin, and total and HMW adiponectin were observed only in the aged group. This might be due to the wider range of fat mass observed in the aged group compared to the adult group (~2–20 g vs. ~6–16 g, respectively). Perhaps, a wider distribution of fat mass amplified existing differences between low and high values, allowing them to become apparent. Other possible reasons for the respective absence/presence of correlations between hormone levels and fat mass in adult/aged mice are intriguing and deserve further investigation. Regarding leptin and insulin, the observed correlations with fat mass were consistent with previous data (Considine et al. 1996; Frederich et al. 1995; Maffei et al. 1995; Carantoni et al. 1998). However, for circulating adiponectin, an inverse relationship with body fat mass is usually observed (Kaser et al. 2008). Also, although mainly related to advanced disease states, adiponectin levels increase in cachexia and may predict mortality (Diez and Iglesias 2010). Thus, the finding of a positive correlation between adiponectin levels and body fat in our study was surprising. One possible reason for our findings might be that the mice studied did not have excessive abundance of body fat. It is possible that the negative association between adiposity and adiponectin levels develops only when increases in body fat are drastic or when insulin sensitivity is severely affected, as seen in ob/ob and db/db mice (Hu et al. 1996; Yamauchi et al. 2001).
The weights of the four WAT depots analyzed were lower in the aged group than in adult mice, with a reciprocal increased trend in protein content. We also recorded a reduction in cell size but increased cell numbers in aged mesenteric and epididymal WAT depots, with similar trends in retroperitoneal and inguinal WAT. These results are consistent with changes observed with age in cell numbers and sizes in WAT of guinea pigs (Pond et al. 1986). Voluntary or involuntary weight loss in humans is also related to decreased adipocyte sizes with no apparent change in adipocyte number (Arner and Spalding 2010). Thus, increased protein content per gram of tissue might be associated with the loss of lipid in wasting adipocytes, resulting in a relative increase in WAT protein content in all depots.
When the protein profiles of WAT depots from 24-month-old mice were resolved and compared to those of 12-month-old mice to investigate the mechanisms leading to fat loss in old age, interesting differences in various proteins were found. Increased levels of ENO, pyruvate dehydrogenase E1 subunit β (PDHE1-B), isocitrate dehydrogenase [NAD+] α (Idh3α), and ATP synthase subunit β in WAT of aged mice might suggest higher rates of glycolysis, conversion of pyruvate to acetyl-CoA, Krebs cycle, and enhanced ATP synthesis via oxidative phosphorylation, respectively. Furthermore, increased levels of epidermal fatty acid binding protein (E-FABP) in aged WAT are consistent with increased lipolysis and decreased lipogenesis (Hertzel et al. 2002), which also agree with the observed age-dependent decreases in adipocyte size and insulin sensitivity. In addition, decreased intensity of two carbonic anhydrase 3 (CA-III) isoforms could reflect a lower rate of anabolic reactions (de novo fatty acid synthesis or glyceroneogenesis), given the proposed function of this enzyme as a bicarbonate donor in these pathways (Herbert et al. 1983; Herbert and Coulson 1984; Coulson and Herbert 1984). Recently, lipolysis, Krebs cycle, the electron transport chain and oxidative phosphorylation were found to be increased in human cancer cachexia (Dahlman et al. 2010). The authors of that study suggested that these changes, which are consistent with our findings in aged WAT, might be due to weight loss and not disease-related. Although more data in WAT are lacking, in agreement with our results, increased levels of ENO, Idh3, ATP synthase subunits, E-FABP and decreased levels of CA-III have been reported for skeletal muscle wasting in aging rats (Donoghue et al. 2010; O’Connell and Ohlendieck 2009; Doran et al. 2009). Thus, increased catabolism might be a general characteristic of very old age leading to tissue wasting. Related to this, lower food intake has been reported for elderly humans as well as aged rats (Frutos et al. 2010; Visvanathan and Chapman 2009), and might represent one cause of activation of catabolism in old age. However, food intake was not measured in our mice.
Proteins with recognized stress-resistant functions were increased in wasting WAT, including Cu/Zn superoxide dismutase and two chaperones: peptidyl-prolyl cis-trans isomerase A and endoplasmic reticulum resident protein 29. Increased levels of these proteins are consistent with higher rates of aerobic glucose oxidation (as suggested by our proteomic data), which would result in elevated ROS production. Given that increased oxidative stress is widely associated with aging (reviewed by Muller et al. 2007a), we evaluated the balance between oxidative stress and stress-resistant proteins, looking for evidence of oxidative damage in the WAT depots. HNE-protein adducts and protein carbonyl content were measured as indicators of lipid peroxidation and protein oxidation, respectively (Halliwell and Whiteman 2004). All depots showed increased lipid peroxidation in aged when compared to adult mice. However, protein oxidation appeared to remain the same or even decrease with age in certain WAT depots. Thus, interestingly, oxidative stress seems to affect lipids and proteins in WAT to different degrees. Inconsistencies between oxidative damage on lipids and proteins in WAT depots have been reported previously (Galinier et al. 2006). After measuring several antioxidant enzymes in ~3-month-old lean Zucker rats, Galinier et al. (2006) concluded that the epididymal depot was subject to higher oxidative stress in the lipophilic compartment than inguinal WAT; and conversely, the hydrophilic compartment remained in a more reduced state in the epididymal than the inguinal WAT depot. These results are consistent with our data, where inguinal WAT showed high protein oxidation compared to the other depots. Also, there was a trend for increased lipid peroxidation in epididymal compared to inguinal adult WAT. However, the differential effects of age on oxidative damage of lipids and proteins in individual WAT depots is novel and worthy of further study. For instance, given the particularly high lipid content of this tissue and the superior diffusion capacity of lipid-derived reactive species, lipid oxidation might be more harmful than protein oxidation in WAT. Also, emerging data suggest a role for protein carbonylation and decarbonylation in cell signaling (Wong et al. 2010), which could play an important role in WAT physiology.
Apart from the observed effects of age, several proteins showed depot-specific differences in intensity in both age groups by proteomics (actin, CA-III, apolipoprotein A-I, hemoglobin β-1, transgelin, myosin regulatory light polypeptide 9—data not shown). Overall, these differences were consistent with our previous analysis of 12-month-old wild-type mice (Sackmann-Sala et al. 2011) and did not show clear changes with wasting.
Finally, it should be noted that although our results showed similar age-related changes in the four studied WAT depots, we did not analyze the different cell populations present in WAT separately. The dynamics of cell populations within WAT are expected to change with age, as has been reported, for example, for preadipocytes (Kirkland et al. 1990). It remains to be evaluated where in WAT the wasting changes take place (adipocytes, preadipocytes, immune cells, endothelium, etc.) and whether depot differences would arise when the effects of wasting are studied individually in each cell population. In addition, the animals in this study were not checked for pathologies. Although most 24-month-old mice are healthy, the presence of age-related diseases that could affect our results cannot be ruled out. Previous studies indicate that at the time of death, old mice mainly display neoplasias (lymphomas, adenocarcinomas of the lung, etc.) and glomerulonephritis (Ikeno et al. 2009). The impact of these pathologies on WAT and wasting warrants further study.
In summary, when adult and aged wild-type mice were compared, there were no differences in body weight or lean mass, but fat mass was significantly decreased in the aged group. Circulating hormone levels (insulin, leptin, and total and HMW adiponectin) were also decreased in the aged group, and WAT depots displayed decreased adipocyte sizes and increased cell numbers. Regarding protein expression profiles, several differences were found in WAT between age groups. Overall, metabolism in wasting WAT might be shifted towards the aerobic oxidation of glucose via glycolysis, Krebs cycle and the electron transport chain, with ATP generation via oxidative phosphorylation. Also, fatty acid and triglyceride synthesis might be decreased and lipolysis and stress resistant proteins increased, consistent with the impaired insulin sensitivity and increased ROS production observed at old age. Lipid peroxidation was elevated in old age, whereas protein oxidation showed no age-specific increase. In general, our data suggest that the individual WAT depots undergo similar changes in their response to insulin and oxidative stress with advancing age/wasting. Further research will help establish causative relations between the changes in the identified protein levels of WAT and the fat loss observed in aged mice and possibly humans. Also, these proteins/pathways might ultimately become putative targets for treatment and/or diagnostic markers of wasting, whether related to age or underlying disease.
Electronic supplementary material
Acknowledgements
This work was supported in part by the State of Ohio’s Eminent Scholar Program that includes a gift from Milton and Lawrence Goll, by the National Institutes of Health (NIH) grants DK075436-01, AG019899-06, and 1P01AG031736-01A1, by the Diabetes Research Initiative and the BioMolecular Innovation and Technology Partnership at Ohio University, and by American Veterans (AMVETS). This work is MIAPE compliant.
Glossary
2DE
2-Dimensional gel electrophoresis
CA-III
Carbonic anhydrase 3
E-FABP
Epidermal fatty acid binding protein
HMW
High molecular weight
HNE
4-Hydroxynonenal
Idh3α
Isocitrate dehydrogenase [NAD+] α
ITT
Insulin tolerance test
LPL
Lipoprotein lipase
MALDI-TOF
Matrix assisted laser desorption/ionization time-of-flight
MS
Mass spectrometry
MS/MS
Tandem MS
PDHE1-B
Pyruvate dehydrogenase E1 subunit β
ROS
Reactive oxygen species
WAT
White adipose tissue
References
- Agustsson T, Ryden M, Hoffstedt J, Harmelen V, Dicker A, Laurencikiene J, Isaksson B, Permert J, Arner P. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007;67(11):5531–5537. doi: 10.1158/0008-5472.CAN-06-4585. [DOI] [PubMed] [Google Scholar]
- Arner P, Spalding KL. Fat cell turnover in humans. Biochem Biophys Res Commun. 2010;396(1):101–104. doi: 10.1016/j.bbrc.2010.02.165. [DOI] [PubMed] [Google Scholar]
- Berryman DE, List EO, Palmer AJ, Chung MY, Wright-Piekarski J, Lubbers E, O’Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):31–40. doi: 10.1093/gerona/glp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berryman DE, List EO, Sackmann-Sala L, Lubbers E, Munn R, Kopchick JJ. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res. 2011;21(3):113–123. doi: 10.1016/j.ghir.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bing C, Trayhurn P. Regulation of adipose tissue metabolism in cancer cachexia. Curr Opin Clin Nutr Metab Care. 2008;11(3):201–207. doi: 10.1097/MCO.0b013e3282f948e2. [DOI] [PubMed] [Google Scholar]
- Bonkowski MS, Pamenter RW, Rocha JS, Masternak MM, Panici JA, Bartke A. Long-lived growth hormone receptor knockout mice show a delay in age-related changes of body composition and bone characteristics. J Gerontol A Biol Sci Med Sci. 2006;61(6):562–567. doi: 10.1093/gerona/61.6.562. [DOI] [PubMed] [Google Scholar]
- Carantoni M, Zuliani G, Volpato S, Palmieri E, Mezzetti A, Vergnani L, Fellin R. Relationships between fasting plasma insulin, anthropometrics, and metabolic parameters in a very old healthy population. Associazione Medica Sabin. Metabolism. 1998;47(5):535–540. doi: 10.1016/S0026-0495(98)90236-0. [DOI] [PubMed] [Google Scholar]
- Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 2010;65(3):242–251. doi: 10.1093/gerona/glp213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HC, Farese RV., Jr Determination of adipocyte size by computer image analysis. J Lipid Res. 2002;43(6):986–989. [PubMed] [Google Scholar]
- Christensen B, Sackmann-Sala L, Cruz-Topete D, Jorgensen JO, Jessen N, Lundby C, Kopchick JJ. Novel serum biomarkers for erythropoietin use in humans: a proteomic approach. J Appl Physiol. 2011;110(1):149–156. doi: 10.1152/japplphysiol.00665.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg A, Young J. The frailty syndrome. Clin Med. 2011;11(1):72–75. doi: 10.7861/clinmedicine.11-1-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- Coulson RA, Herbert JD. A role for carbonic anhydrase in intermediary metabolism. Ann N Y Acad Sci. 1984;429:505–515. doi: 10.1111/j.1749-6632.1984.tb12379.x. [DOI] [PubMed] [Google Scholar]
- Cruz-Topete D, Christensen B, Sackmann-Sala L, Okada S, Jorgensen JO, Kopchick JJ. Serum proteome changes in acromegalic patients following transsphenoidal surgery: novel biomarkers of disease activity. Eur J Endocrinol. 2011;164(2):157–167. doi: 10.1530/EJE-10-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Topete D, Jorgensen JO, Christensen B, Sackmann-Sala L, Krusenstjerna-Hafstrom T, Jara A, Okada S, Kopchick JJ (2011b) Identification of new biomarkers of low-dose GH replacement therapy in GH-deficient patients. J Clin Endocrinol Metab 96(7):2089–2097 [DOI] [PMC free article] [PubMed]
- Cruz-Topete D, List EO, Okada S, Kelder B, Kopchick JJ. Proteomic changes in the heart of diet-induced pre-diabetic mice. J Proteomics. 2011;74(5):716–727. doi: 10.1016/j.jprot.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlman I, Mejhert N, Linder K, Agustsson T, Mutch DM, Kulyte A, Isaksson B, Permert J, Petrovic N, Nedergaard J, Sjolin E, Brodin D, Clement K, Dahlman-Wright K, Ryden M, Arner P. Adipose tissue pathways involved in weight loss of cancer cachexia. Br J Cancer. 2010;102(10):1541–1548. doi: 10.1038/sj.bjc.6605665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das SK, Eder S, Schauer S, Diwoky C, Temmel H, Guertl B, Gorkiewicz G, Tamilarasan KP, Kumari P, Trauner M, Zimmermann R, Vesely P, Haemmerle G, Zechner R, Hoefler G (2011) Adipose triglyceride lipase contributes to cancer-associated cachexia. Science. doi:10.1126/science.1198973 [DOI] [PubMed]
- DeFronzo RA. Glucose intolerance and aging. Diabetes Care. 1981;4(4):493–501. doi: 10.2337/diacare.4.4.493. [DOI] [PubMed] [Google Scholar]
- Diez JJ, Iglesias P. The role of the novel adipocyte-derived protein adiponectin in human disease: an update. Mini Rev Med Chem. 2010;10(9):856–869. doi: 10.2174/138955710791608325. [DOI] [PubMed] [Google Scholar]
- Ding J, Kopchick JJ (2011) Plasma biomarkers of mouse aging. Age (Dordr) 33(3):291–307 [DOI] [PMC free article] [PubMed]
- Ding J, Berryman DE, Kopchick JJ (2011) Plasma proteomic profiles of bovine growth hormone transgenic mice as they age. Transgenic Res. doi:10.1007/s11248-011-9499-5 [DOI] [PMC free article] [PubMed]
- Donoghue P, Staunton L, Mullen E, Manning G, Ohlendieck K. DIGE analysis of rat skeletal muscle proteins using nonionic detergent phase extraction of young adult versus aged gastrocnemius tissue. J Proteomics. 2010;73(8):1441–1453. doi: 10.1016/j.jprot.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Doran P, Donoghue P, O’Connell K, Gannon J, Ohlendieck K. Proteomics of skeletal muscle aging. Proteomics. 2009;9(4):989–1003. doi: 10.1002/pmic.200800365. [DOI] [PubMed] [Google Scholar]
- Dorner TE, Rieder A (2011) Obesity paradox in elderly patients with cardiovascular diseases. Int J Cardiol [DOI] [PubMed]
- Fouladiun M, Korner U, Bosaeus I, Daneryd P, Hyltander A, Lundholm KG. Body composition and time course changes in regional distribution of fat and lean tissue in unselected cancer patients on palliative care—correlations with food intake, metabolism, exercise capacity, and hormones. Cancer. 2005;103(10):2189–2198. doi: 10.1002/cncr.21013. [DOI] [PubMed] [Google Scholar]
- Frederich RC, Hamann A, Anderson S, Lollmann B, Lowell BB, Flier JS. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat Med. 1995;1(12):1311–1314. doi: 10.1038/nm1295-1311. [DOI] [PubMed] [Google Scholar]
- Frutos MG, Pistell PJ, Ingram DK, Berthoud HR (2010) Feed efficiency, food choice, and food reward behaviors in young and old Fischer rats. Neurobiol Aging. doi:10.1016/j.neurobiolaging.2010.09.006 [DOI] [PMC free article] [PubMed]
- Galinier A, Carriere A, Fernandez Y, Caspar-Bauguil S, Periquet B, Periquet A, Penicaud L, Casteilla L. Site specific changes of redox metabolism in adipose tissue of obese Zucker rats. FEBS Lett. 2006;580(27):6391–6398. doi: 10.1016/j.febslet.2006.10.052. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142(2):231–255. doi: 10.1038/sj.bjp.0705776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert JD, Coulson RA. A role for carbonic anhydrase in de novo fatty acid synthesis in liver. Ann N Y Acad Sci. 1984;429:525–527. doi: 10.1111/j.1749-6632.1984.tb12381.x. [DOI] [PubMed] [Google Scholar]
- Herbert JD, Coulson RA, Hernandez T. Inhibition of pyruvate carboxylation in alligators and chameleons by carbonic anhydrase inhibitors. Comp Biochem Physiol A Mol Integr Physiol. 1983;75A(2):185–192. [Google Scholar]
- Hertzel AV, Bennaars-Eiden A, Bernlohr DA. Increased lipolysis in transgenic animals overexpressing the epithelial fatty acid binding protein in adipose cells. J Lipid Res. 2002;43(12):2105–2111. doi: 10.1194/jlr.M200227-JLR200. [DOI] [PubMed] [Google Scholar]
- Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82(963):2–8. doi: 10.1136/pgmj.2005.037564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271(18):10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Ikeno Y, Hubbard GB, Lee S, Cortez LA, Lew CM, Webb CR, Berryman DE, List EO, Kopchick JJ, Bartke A. Reduced incidence and delayed occurrence of fatal neoplastic diseases in growth hormone receptor/binding protein knockout mice. J Gerontol A Biol Sci Med Sci. 2009;64(5):522–529. doi: 10.1093/gerona/glp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser S, Tatarczyk T, Stadlmayr A, Ciardi C, Ress C, Tschoner A, Sandhofer A, Paulweber B, Ebenbichler CF, Patsch JR. Effect of obesity and insulin sensitivity on adiponectin isoform distribution. Eur J Clin Invest. 2008;38(11):827–834. doi: 10.1111/j.1365-2362.2008.02028.x. [DOI] [PubMed] [Google Scholar]
- Kirkland JL, Hollenberg CH, Gillon WS. Age, anatomic site, and the replication and differentiation of adipocyte precursors. Am J Physiol. 1990;258(2 Pt 1):C206–210. doi: 10.1152/ajpcell.1990.258.2.C206. [DOI] [PubMed] [Google Scholar]
- Kizer JR, Arnold AM, Strotmeyer ES, Ives DG, Cushman M, Ding J, Kritchevsky SB, Chaves PH, Hirsch CH, Newman AB. Change in circulating adiponectin in advanced old age: determinants and impact on physical function and mortality. The Cardiovascular Health Study All Stars Study. J Gerontol A Biol Sci Med Sci. 2010;65(11):1208–1214. doi: 10.1093/gerona/glq122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Kim SR, Yoo SJ, Hong OK, Son HS, Chang SA. The relationship between adipokines, metabolic parameters and insulin resistance in patients with metabolic syndrome and type 2 diabetes. J Int Med Res. 2009;37(6):1803–1812. doi: 10.1177/147323000903700616. [DOI] [PubMed] [Google Scholar]
- List EO, Berryman DE, Palmer AJ, Qiu L, Sankaran S, Kohn DT, Kelder B, Okada S, Kopchick JJ. Analysis of mouse skin reveals proteins that are altered in a diet-induced diabetic state: a new method for detection of type 2 diabetes. Proteomics. 2007;7(7):1140–1149. doi: 10.1002/pmic.200600641. [DOI] [PubMed] [Google Scholar]
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, et al. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–1161. doi: 10.1038/nm1195-1155. [DOI] [PubMed] [Google Scholar]
- Miller SL, Wolfe RR. The danger of weight loss in the elderly. J Nutr Health Aging. 2008;12(7):487–491. doi: 10.1007/BF02982710. [DOI] [PubMed] [Google Scholar]
- Morley JE. Weight loss in older persons: new therapeutic approaches. Curr Pharm Des. 2007;13(35):3637–3647. doi: 10.2174/138161207782794149. [DOI] [PubMed] [Google Scholar]
- Muller FL, Lustgarten MS, Jang Y, Richardson A, Remmen H. Trends in oxidative aging theories. Free Radic Biol Med. 2007;43(4):477–503. doi: 10.1016/j.freeradbiomed.2007.03.034. [DOI] [PubMed] [Google Scholar]
- Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, Remmen H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1159–1168. doi: 10.1152/ajpregu.00767.2006. [DOI] [PubMed] [Google Scholar]
- Narimiya M, Azhar S, Dolkas CB, Mondon CE, Sims C, Wright DW, Reaven GM. Insulin resistance in older rats. Am J Physiol. 1984;246(5 Pt 1):E397–404. doi: 10.1152/ajpendo.1984.246.5.E397. [DOI] [PubMed] [Google Scholar]
- O’Connell K, Ohlendieck K. Proteomic DIGE analysis of the mitochondria-enriched fraction from aged rat skeletal muscle. Proteomics. 2009;9(24):5509–5524. doi: 10.1002/pmic.200900472. [DOI] [PubMed] [Google Scholar]
- Okada S, List E, Sankaran S, Kopchick J. Plasma protein biomarkers correlated with the development of diet-induced type 2 diabetes in mice. Clin Proteomics. 2010;6(1–2):6–17. doi: 10.1007/s12014-009-9040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA, Thompson MC, Sadler D. The effects of age, dietary restriction, exercise and maternity on the abundance and volume of adipocytes in twelve adipose depots of adult guinea-pigs. Br J Nutr. 1986;56(1):29–48. doi: 10.1079/BJN19860083. [DOI] [PubMed] [Google Scholar]
- Qiu L, List EO, Kopchick JJ. Differentially expressed proteins in the pancreas of diet-induced diabetic mice. Mol Cell Proteomics. 2005;4(9):1311–1318. doi: 10.1074/mcp.M500016-MCP200. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol Rev. 2006;86(2):651–667. doi: 10.1152/physrev.00019.2005. [DOI] [PubMed] [Google Scholar]
- Sackmann-Sala L, Ding J, Frohman LA, Kopchick JJ. Activation of the GH/IGF-1 axis by CJC-1295, a long-acting GHRH analog, results in serum protein profile changes in normal adult subjects. Growth Horm IGF Res. 2009;19(6):471–477. doi: 10.1016/j.ghir.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackmann-Sala L, Berryman DE, Munn RD, Lubbers ER, Kopchick JJ (2011) Heterogeneity among white adipose tissue depots in male C57BL/6J mice. Obesity (Silver Spring): Epub ahead of print [DOI] [PMC free article] [PubMed]
- Silver AJ, Guillen CP, Kahl MJ, Morley JE. Effect of aging on body fat. J Am Geriatr Soc. 1993;41(3):211–213. doi: 10.1111/j.1532-5415.1993.tb06693.x. [DOI] [PubMed] [Google Scholar]
- Singh PN, Haddad E, Tonstad S, Fraser GE. Does excess body fat maintained after the seventh decade decrease life expectancy? J Am Geriatr Soc. 2011;59(6):1003–1011. doi: 10.1111/j.1532-5415.2011.03419.x. [DOI] [PubMed] [Google Scholar]
- Tchoukalova YD, Koutsari C, Votruba SB, Tchkonia T, Giorgadze N, Thomou T, Kirkland JL, Jensen MD. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring) 2010;18(10):1875–1880. doi: 10.1038/oby.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorner MO. Statement by the Growth Hormone Research Society on the GH/IGF-I axis in extending health span. J Gerontol A Biol Sci Med Sci. 2009;64(10):1039–1044. doi: 10.1093/gerona/glp091. [DOI] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvanathan R, Chapman IM. Undernutrition and anorexia in the older person. Gastroenterol Clin North Am. 2009;38(3):393–409. doi: 10.1016/j.gtc.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Wajchenberg BL, Giannella-Neto D, Silva ME, Santos RF. Depot-specific hormonal characteristics of subcutaneous and visceral adipose tissue and their relation to the metabolic syndrome. Horm Metab Res. 2002;34(11–12):616–621. doi: 10.1055/s-2002-38256. [DOI] [PubMed] [Google Scholar]
- Wong CM, Marcocci L, Liu L, Suzuki YJ. Cell signaling by protein carbonylation and decarbonylation. Antioxid Redox Signal. 2010;12(3):393–404. doi: 10.1089/ars.2009.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7(8):941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.