PET Amyloid-Beta Imaging in Preclinical Alzheimer’s Disease (original) (raw)
. Author manuscript; available in PMC: 2013 Mar 1.
Published in final edited form as: Biochim Biophys Acta. 2011 Nov 12;1822(3):370–379. doi: 10.1016/j.bbadis.2011.11.005
Alzheimer's disease (AD) is the leading cause of dementia, accounting for 60–70% of all cases [1]. The need for effective therapies for AD is great. Current approaches, including cholinesterase inhibitors and N-methyl-D-aspartate (NMDA) receptor antagonists, are symptomatic treatments for AD but do not prevent disease progression. Many diagnostic and therapeutic approaches to AD are currently changing due to the knowledge that underlying pathology starts 10 to 20 years before clinical signs of dementia appear [2]. New therapies which focus on prevention or delay of the onset or cognitive symptoms are needed. Recent advances in the identification of AD biomarkers now make it possible to detect AD pathology in the preclinical stage of the disease, in cognitively normal (CN) individuals; this biomarker data should be used in the selection of high-risk populations for clinical trials. In vivo visualization of AD neuropathology and biological, biochemical or physiological confirmation of the effects of treatment likely will substantially improve development of novel pharmaceuticals. Positron emission tomography (PET) is the leading neuroimaging tool to detect and provide quantitative measures of AD amyloid pathology in vivo at the early stages and follow its course longitudinally.
Aβ metabolism
Although the exact cause-and-effect relations in AD are not well understood, several potential underlying abnormalities have been suggested including increased production, misfolding and deposition of proteins; disruption of receptor and neurotransmitter state; changes in synaptic density and functioning; and eventually degeneration and death of neurons with resultant brain atrophy [3–6].
Pathologically, AD primarily is characterized by the presence of two abnormal proteins in the aggregated state: extracellular plaques composed of beta-amyloid (Aβ) and intraneuronal neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau [7]. The Aβ peptide is cleaved by different secretases from the transmembrane protein amyloid precursor protein (APP). When normally soluble Aβ proteins reach a critical concentration, they become insoluble, misfold, and aggregate in the form of Aβ plaques. Distinct plaque subtypes have been identified: those with low (diffuse plaques) and high (cored or neuritic plaques) proportion of fibrillar components [8]. Insoluble Aβ exceeds soluble forms of Aβ by a factor of about 100-fold in AD brain [9]. Studies of immunotherapies in transgenic mice [10, 11] and autopsy studies of humans treated with active immunization in the AN-1792 trial [12–14] indicate that Aβ in fibrillar plaques can be mobilized and cleared.
Plaques are composed of insoluble Aβ peptides, mostly 42 amino acids in length (Aβ-42) [15]. It is possible that the small aggregated oligomeric forms of Aβ-42, rather than fibrils or plaques, are the key pathological substrates [16].
It should be noted that in rare cases of AD, genetic alterations increase the production of Aβ [17]. However, impaired clearance of Aβ may cause late-onset AD through interactions with apolipoprotein E (APOE) ε4, decreased catabolism of Aβ through reduced proteolysis, impaired transport across the blood-brain barrier, or impaired CSF transport [18].
Aβ cascade hypothesis
Characterization of the initial deposition of Aβ plaques is important to improve our understanding of the pathology of early AD. The amyloid-cascade hypothesis suggests that APP mismetabolism and subsequent Aβ aggregation are the primary events driving AD pathogenesis [19–21]. High levels of Aβ subsequently lead to a series of downstream pathological events, including the production of extensive intracellular NFT deposits, inflammation, oxidative stress, excitotoxicity, loss of synaptic connections, and cell death, which cause the clinical manifestations of AD. Multiple causative factors may be involved [2, 22–24]. Mutations in the Aβ precursor protein gene on chromosome 21, all lying in or near the Aβ peptide region, cause early-onset, autosomal dominant familial forms of AD [25–27]. Presenilin-1 [28] and presenilin-2 [29] are other two genes that encode highly homologous transmembrane proteins, in which multiple mutations have been identified in familial early-onset and late-onset AD [30]. In addition to potentially harmful effects of Aβ for brain cells [31], increased synaptic activity can elevate soluble Aβ levels [32] and neural activity can change levels of APP [33], which suggests that Aβ production and soluble levels may be in a dynamic equilibrium state.
A close relationship between synaptic activity, brain metabolism and Aβ deposition has been recently advocated by the results of a study which observed a high spatial correlation between the pattern of Aβ deposition in patients with dementia of Alzheimer type (DAT) patients and the pattern of aerobic glycolysis measured using combined glucose and oxygen metabolism PET studies in healthy young adults [34]. These data indicate that when Aβ accumulates in the human brain in AD, it does so in a distribution that closely mirrors that of elevated aerobic glycolysis in the resting state of healthy young adults [34, 35]. Strong correlation between aerobic glycolysis and Aβ deposition was demonstrated not only in DAT patients but also in CN individuals with abnormally elevated levels of Aβ, which indicate that these CN individuals may be at the earlier stage of pathological processes seen in the DAT group [34], the stage which is considered to represent preclinical AD [36].
Even if Aβ is not the only or even the main causal event in AD pathology, the deposition of Aβ is likely important for signifying the beginning of the pathological cascade. Since all young healthy persons and many CN older individuals have no evidence of Aβ deposition, the conversion of a non-demented person from no evidence of Aβ plaques to Aβ deposits in a cerebral distribution corresponding to that seen in advanced DAT suggests a pathological event. We believe that the pathological cascade may eventually become irreversible and independent of the availability and level of Aβ, however we do not know at what stage of AD this irreversibility may be achieved. Thus, it is critical to find better tools for determination of optimal timing for interventions aimed on decreasing Aβ level and especially those which may prevent irreversibility and further progression of pathological cascade.
Biomarkers of AD
Biomarkers provide unique, biological measure of the underlying pathology independent of clinical signs and neuropsychological characteristics of AD, and identification of reliable biomarkers is critical for evaluation of the preclinical/asymptomatic state of the AD. To date, the most widely studied and best validated biomarkers include cerebrospinal fluid (CSF) assays of Aβ and tau, and amyloid imaging with N-methyl-[11C]2-(4=-methylaminophenyl)-6-hydroxybenzothiazole (Pittsburgh Compound B, or [11C]PIB) [2, 37–39]. In addition, decreased glucose metabolism in temporal and parietal cortex is considered as a biomarker of synaptic dysfunction (commonly seen in the symptomatic phase of AD). Similarly, brain atrophy in the medial temporal lobes, paralimbic, temporal and parietal cortex on structural MRI is a biomarker of AD-related neurodegeneration, also commonly seen during the symptomatic phase of AD. Recently a biomarker model has been recently proposed which suggests an ordered manner of abnormalities of these biomarkers which parallels the hypothetical pathophysiological sequence of AD and is particularly relevant to tracking the preclinical stages of AD [39, 40].
Levels of CSF Aβ42 are commonly decreased in patients with AD [41]. In a study comparing fluid and imaging measures of potential preclinical biomarkers of AD pathology, a robust relationship was observed between cortical [11C]PIB binding and levels of CSF Aβ42 in CN individuals [42, 43]. Individuals with high cortical Aβ as detected by [11C] PIB PET had low CSF Aβ42 whereas those without cortical Aβ had high Aβ42. These findings suggest that a low CSF Aβ42 level is a sensitive marker of Aβ deposition in the brain regardless of the state of AD.
It should be noted that decreased CSF Aβ42 levels have also been reported in frontotemporal dementia, vascular dementia, Creutzfeldt-Jacob disease and dementia with Lewy bodies [44–46]. Potential limitation of AD studies that have used CSF Aβ42 is the lack of standardization for Aβ quantification. Little is known about the CSF Aβ42 turnover and clearance in normal aging, and known circadian fluctuations of CSF Aβ42 levels [47] may possibly contribute to the variability of results. Levels of CSF Aβ42 do not correlate well with AD duration or severity [48], which is consistent with Aβ imaging data of little changes in Aβ accumulation in clinical AD [49] and may suggest that amyloid pathology occurs very early in course of the disease but may have stabilized by the clinical manifestations of DAT.
Increased levels of CSF tau may indicate neuronal injury from multiple causes and were seen in AD patients [41, 45, 48, 50–52] as well as in frontotemporal dementia, stroke and Creutzfeldt-Jacob disease [51]. In preclinical AD, CSF tau levels are correlated with the amount of Aβ deposition [43]. Higher concentrations of CSF tau are associated with greater cognitive impairment in preclinical and clinical AD [53]. In AD, tau undergoes abnormal hyperphosphorylation and phosphorylated tau (p-tau) may offer equivalent if not better diagnostic utility for AD than total tau. Various phosphorylated epitopes may be effective in differentiating AD patients from controls [54], and p-tau231 appears to provide diagnostic specificity for AD and to improve the differentiation between AD and frontotemporal dementia [55], while p-tau181 helps the differentiation between AD and dementia with Lewy bodies [54].
The ratios of CSF tau/Aβ42 and p-tau/Aβ42 in CN individuals strongly predict progression to clinical state of AD. Over 3–4 years of follow-up, 70% of individuals with elevated tau/Aβ42 ratios became clinically positive in one study [52], and another study demonstrated that all individuals who converted to mild cognitive impairment had elevated tau/Aβ42 ratios [56].
Aβ tracers
With the development of positron emission tomography (PET) radiotracers with high in vivo binding to Aβ plaques it is now possible to quantify pathological changes in the human brain that were previously restricted to post-mortem studies. Ideally, Aβ tracers should: 1) effectively image brain Aβ deposition; 2) have good reproducibility across many subjects and clinical settings; and 3) be widely accessible and appropriate for the particular task [57]. Only a few current tracers satisfy these requirements; however, new tracers for Aβ imaging are under development.
As the comparative review of chemistry of Aβ–specific 11C and 18F PET radiopharmaceuticals has been recently provided by others [58, 59], we will present very briefly characteristics of known radiotracers and focus our discussion on the existing PET imaging data in preclinical AD. [18F]FDDNP [60] was the first reported PET-tracer to image AD pathology in vivo. A higher retention of the compound was demonstrated in hippocampus, amygdala and enthorhinal cortex of individuals with symptomatic AD compared with CN persons [61]. However, [18F]FDDNP has relatively high non-specific binding although it may also bind to tau protein [62–64]. The stilbene 4-N-[11C-methyl]amino-4'-hydroxystilbene ([11C] SB-13) has been proposed as a compound with high affinity for Aβ, high initial brain uptake and relatively rapid washout from normal rat brain after an intravenous injection, however it has relatively higher uptake in healthy individuals [65, 66].
Recently, several 18F-labeled tracers in addition to FDDNP were designed and are currently under study including flobetapir ([18F]AV-45), flutemetamol ([18F]GE067), florbetaben ([18F] BAY94–9172) [63, 67–69]. [18F] BAY94–9172 demonstrated neocortical binding in AD patients which was greater in precuneus/posterior cingulate and frontal cortex than in lateral temporal and parietal cortex, with relative sparing of occipital, sensorimotor and medial temporal cortex. At 90–120 min after injction, higher neocortical SUVR was observed in AD patients compared to healthy controls or patients with frontotemporal lobar degeneration. Visual interpretation was 100% sensitive and 90% specific for detection of AD [67]. It has been suggested that further validation of [18F] BAY94–9172 is required in order to evaluate better the kinetics and metabolism of the tracer and determine appropriate quantification method [67].
[18F] AV-45 has high binding affinity, as demonstrated by in vitro binding studies using AD brain homogenates; high selective Aβ plaque labeling, as demonstrated by in vitro autoradiography using postmortem AD brain sections; and excellent brain penetration and rapid kinetics in healthy mice and nonhuman primates [70–72]. The radiochemistry of [18F] AV-45 has been optimized to make this procedure simpler and more convenient and suitable for routine production and distribution of this radiopharmaceutical [71, 72]. In humans, [18F] AV-45 is well tolerated and it shows significant discrimination between AD patients and healthy controls [73]. It is rapidly cleared from circulation, and maximum uptake occurs approximately 30 minutes after injection and remains essentially unchanged for the subsequent 60 minutes [73], providing enough time to obtain a 10-min image. The dosimetry of [18F] AV-45 is suitable for clinical and research applications including longitudinal studies of Aβ accumulation [74]. No evidence of elevated Aβ deposition with [18F] AV-45 was demonstrated in CN individuals within the age range of 18 and 50 years, regardless of APOE genotype [75]. [18F] AV-45 imaging in multicenter study was correlated with the presence and density of Aβ at autopsy, and more studies are ongoing to evaluate this marker in the clinical diagnosis of AD and for the prediction of progression to dementia [75]. Aβ tracers also have been proposed for single photon emission tomography, including 123I-IMPY and 125I aurone derivatives [76–79].
[11C] PIB PET
[11C]PIB currently is the most studied and used tracer for PET imaging of cerebral Aβ pathology in vivo [80, 81]. Significantly higher cerebral binding of [11C]PIB has been demonstrated in a specific pattern in the individuals with symptomatic AD patients compared with CN older adults [49, 82–84] (Figure 1). [11C] PIB is selective for fibrillar Aβ with high affinity to a single binding site in homogenates from AD brains [85, 86] and minimal binding to cerebellum. Therefore, standard uptake values, corrected by using the cerebellum as reference region, provide reproducible results with a scanning time of 60–90 min [85]. Methodology and radiation dosimetry for quantitation of [11C] PIB uptake has been documented [87, 88]. [11C] PIB brain uptake may be quantified using distribution volume ratios (DVR) or binding potentials (BP) obtained with the Logan graphic method using the cerebellum as the reference region, or it may be assessed using some standardized uptake value ratio (SUVR), which is analyzed manually or with automatic algorithm and usually represents the ratio of [11C] PIB accumulation in particular cortical region to [11C] PIB retention in the whole brain [84, 89–92]. Recently, an image-derived input function approach was proposed for quantitative assessment of [11C] PIB retention without arterial sampling [93] and other techniques are under development.
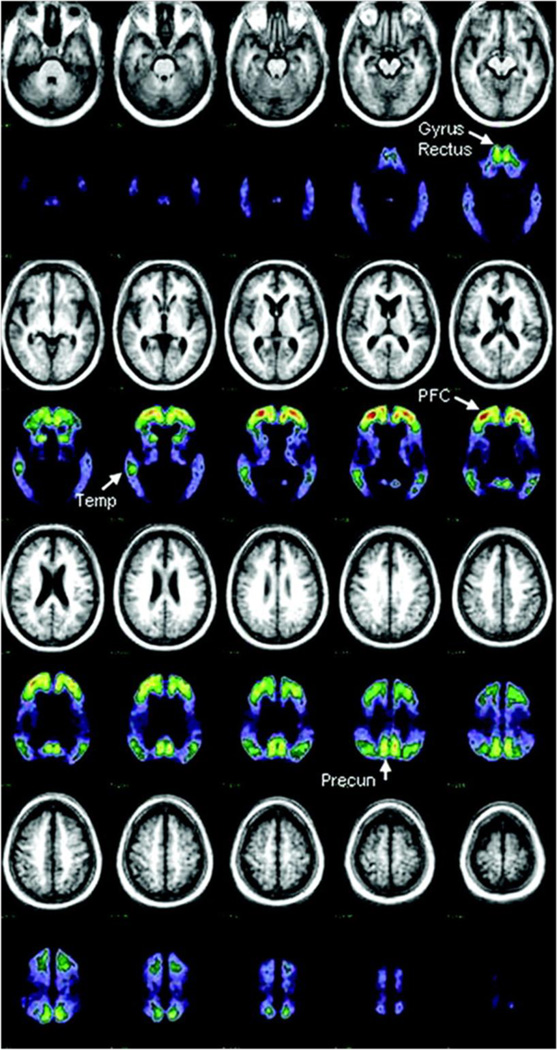
Figure 1.
Mean MRI and PET [11C]PIB distribution in a standard atlas coordinate system from 10 subjects with dementia of the Alzheimer type. PET data represent [11C]PIB activity in the late (30 to 60 minutes after injection) distribution and has been normalized to standardize display and increase contrast. Brain areas used to detect raised [11C] PIB uptake in nondemented subjects are indicated with arrows. PFC = prefrontal cortex; Temp = temporal cortex; Precun = precuneus region. Reproduced with permission from [84].
Regional analysis is usually provided with manually defined regions of interests (ROIs) using co-registered MR image [84, 85, 94]; and semi-automatic and automatic [95] or voxel-wise (SPM) analysis may also be used [90]. Various ROIs and global means of representative cortical regions are used by different centers. In our university, we define mean cortical binding potential (MCBP) calculated from the mean of the binding potential values in the prefrontal cortex, gyrus rectus, lateral temporal, and precuneus regions [42, 84, 96, 97]. Others use mean cortical DVR averaged from frontal, anterior cingulate, precuneus, lateral temporal, and parietal cortex and striatum [94] or calculate mean DVR from manually drawn orbitofrontal, prefrontal, superior frontal, parietal, lateral temporal, occipital, and anterior and posterior cingulate regions [98]. Average cortical SUVR may also be calculated from area-weighted mean of frontal, superior parietal, lateral temporal, lateral occipital, and anterior and posterior cingulate regions [95]. Although there is some consensus between various centers regarding these regions, variations in ROIs and protocols for manually drawn regions make cross-center comparisons difficult. A standardized, automated approach with full, accurate segmentation of the brain is still needed in the field. A promising approach is the use of the FreeSurfer (http://surfer.nmr.mgh.harvard.edu), a set of automated tools for reconstruction of the brain’s cortical surface from structural MRI data, to create multiple standard regions of interests and facilitate the quantitative regional analysis of PET images. FreeSurfer automatically segments and parcellates T1-weighted brain MR images [99–101] and create distinct regions in cortical and subcortical gray matter, which then can be applied to co-rewho had [11C] PIB scanning prior to shunt placement [110]. Excellent agreement existed between levels of [11C] PIB PET uptake and Aβ quantitation in the post-mortem regions [108]. The biopsy study showed high correlation with Pearson r = 0.85 and very good agreement in 9 of 10 biopsies [110]. However, in one biopsy there was evidence of Aβ by histology but when the [11C] PIB PET scan was done (after a period of 20 months) there was no elevated [11C] PIB uptake in the brain region. There is no clear explanation for this apparent “false-negative” [11C] PIB PET scan, in view of the excellent correlation of [11C] PIB and Aβ in the other subjects.
[11C] PIB has been shown to bind specifically to Aβ-40 and Aβ-42 synthetic fibrils and insoluble Aβ plaques containing Aβ-42 and Aβ-40 found in AD brain [108, 111]. In contrast, [11C] PIB does not bind appreciably to soluble Aβ and probably does not bind to oligomeric forms of Aβ nor to nonfibrillar plaques until they reach some critical size (yet to be determined). [11C] PIB binding requires an extended Aβ pleated sheet structure found in Aβ fibrils and plaques in order to bind with high affinity. The binding of the tracer [11C]PIB to NFTs has been previously evaluated and is regarded as negligible [112, 113]. However, Johnson et al. [114] demonstrated substantially increased [11C]PIB binding in the occipital cortex of patients with cerebral amyloid angiopathy. 18F-labeled PIB analogues have been synthesized recently and are under experimental evaluations now [115, 116].
Aβ imaging in preclinical AD
The concept of preclinical AD postulates that AD lesions accumulate in the brain for years prior to appearance of cognitive deficits or symptoms of dementia [36]. Preclinical AD assumes that AD pathology in CN individuals ultimately culminates in progressive neuronal deterioration that results in the clinical manifestations of DAT, although the time to DAT may differ depending on reserve capacities of an individual [117–119]. It is possible that some individuals with presumptive preclinical AD may never develop DAT, no matter how long they live.
Postmortem morphometric analysis of lesion densities in individuals age 54 to 89 years of age who were CN in life or who had very mild DAT or advanced DAT found that large densities of senile plaques and Aβ immunohistochemistry were present in the neocortex in all demented cases and in 31% of CN individuals, while NFTs were restricted in the CN individuals to the parahippocampal gyrus and hippocampal field CA1 [120]. These observations were extended in subsequent reports [4, 121, 122], concluding that the cerebral deposition of amyloid in the form of Aβ plaques appears to accelerate an age-related tauopathy and that the AD disease process begins well before clinical detection of dementia. Seven of 26 CN individuals older than age 75 had extensively distributed neocortical diffuse and neuritic senile plaques in densities sufficient for a neuropathological diagnosis of AD, which was interpreted as a preclinical stage of AD [4].
Current recommendations from the National Institute on Aging and Alzheimer’s Association workgroups are that both the underlying pathological and pathophysiological process of AD and clinical symptoms should be best conceptualized as a continuum or a trajectory, and that these processes may evolve in parallel but temporally offset trajectories [39]. According to this concept, three staging categories were defined for preclinical AD research. Stage 1, “the stage of asymptomatic cerebral amyloidosis”, implies biomarker evidence of Aβ accumulation with elevated PET tracer retention and/or low CSF Aβ42, but no detectable evidence of additional brain alterations suggestive of neurodegeneration or behavioral impairment. Stage 2, “amyloid positivity + evidence of synaptic dysfunction and/or early neurodegeneration”, includes besides amyloid accumulation, presence of one or more markers of downstream neuronal injury related to AD pathology, including elevated CSF tau or phospho-tau, decreased FDG uptake and cortical thinning/atrophy in AD-prone cortical regions. Stage 3, “amyloid positivity + evidence of neurodegeneration + subtle cognitive decline”, is characterized, besides evidence of Aβ accumulation and neurodegeneration, by appearance of subtle cognitive decline and approaching the border zone with the proposed clinical criteria for mild cognitive impairment [39].
Imaging and molecular biomarkers for AD now identify in vivo correlates of neuropathological AD and may be used as markers of preclinical AD. Abnormally elevated [11C]PIB uptake in what has been considered “healthy aging” has been consistently demonstrated from very early [11C]PIB studies and it is likely an important in vivo pathological hallmark of preclinical AD. In the initial report by Klunk et al. [80], elevated PIB uptake was seen in 1 of the 6 CN older controls. In a subsequent study [84], 4 of the 23 subjects over 65 yrs had elevated PIB uptake. Later Pike et al. [123] identified 22% of the 32 CN controls and Aizenstein et al. [94] reported 21% of 43 clinically unimpaired elderly persons as having Aβ plaques. Higher frequency (30–33%) of elevated [11C]PIB BP in CN adults especially in older individuals are observed in recent studies involving larger cohorts, and these percentages are comparable to age-related frequency of neuropathological AD on postmortem examination of CN adults [96, 124, 125].
The spatial distribution of [11C] PIB uptake in CN older individuals is similar to AD individuals and is elevated in the posterior cingulate, precuneus, gyrus rectus, orbitofrontal, prefrontal and lateral temporal cortex with relatively spared occipital and sensorimotor areas (Figure 2) [80, 84, 94, 123, 126]. The highest [11C] PIB BP of all gray matter regions is noted in the posterior cingulate, precuneus and prefrontal cortex [84, 94, 95, 127]. CN individuals demonstrate similar or lower levels of [11C] PIB uptake compared to mild cognitive impairment and DAT [84, 95].
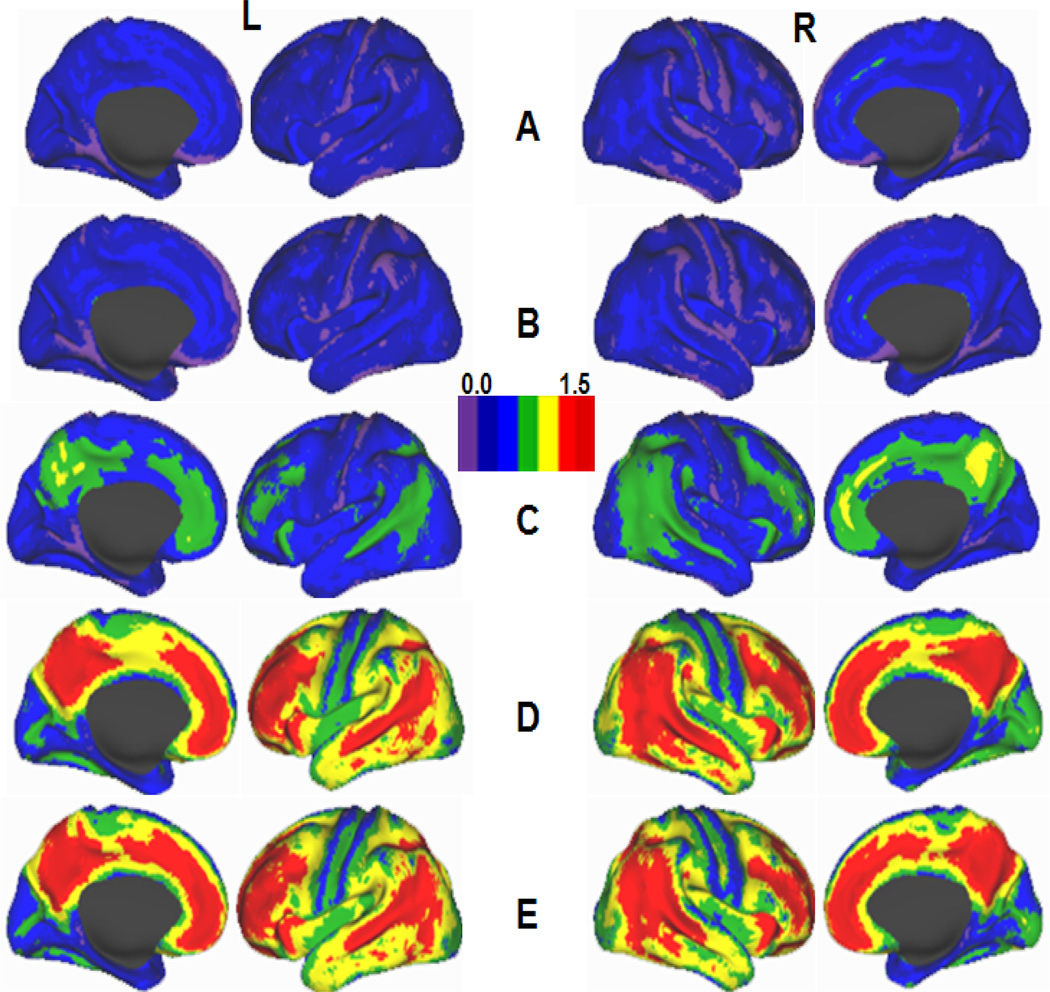
Figure 2.
Schematic maps showing [11C] PIB BP distribution on lateral and medial cortical surfaces of the left (L) and right (R) hemispheres of the human brain. A, Healthy young (< 50 y. o) adults; **B–D**, Cognitively normal older (> 50 y. o.) adults with low (B), moderate (C) and high (D) Aβ deposition; E, Individuals with dementia of Alzheimer’s type (modified from Vlassenko et al. 2010 with permission).
Autosomal dominant AD attracts increasing attention, because it allows evaluation of Aβ accumulation in young and middle-aged adults with known mutation years or decades before clinical manifestations. [11C] PIB PET studies have revealed evidence of Aβ regional deposition especially in precuneus, posterior cingulate and prefrontal cortex, and in stratum, in carriers, including those who were up to 10 years younger than the age of onset for their family [128– 130].
In longitudinal studies using [11C] PIB as an indicator for Aβ accumulation, no or little increase in tracer uptake was found during 1–3 years of follow-up in individuals with mild cognitive impairment and DAT [82, 131, 132]. These findings indicate that brain Aβ accumulates more rapidly in the early phases of AD and only very slowly in the more advanced stages of the disease. Recent longitudinal [11C] PIB studies demonstrated increase of Aβ accumulation over time in CN individuals with elevated baseline levels of Aβ [95, 98].
Sojkova et al [98] reported longitudinal [11C] PIB DVR data in 14 older individuals with minimal and 10 with elevated initial Aβ accumulation. The group with elevated mean cortical DVR showed significant 2.3% increase in Aβ accumulation compared to baseline, and all 4 individuals with three [11C] PIB studies demonstrated continuous progression, which was linear in 3 cases of 4. Regionally, significant increases in [11C] PIB DVR were observed in the prefrontal, superior frontal, parietal, lateral temporal, occipital, and anterior cingulate cortex, and in the combined group of CN adults the highest annual increase was demonstrated in posterior cingulate cortex. None of these participants met the diagnostic criteria for mild cognitive impairment (MCI), however 4 had CDR 0.5, and they all demonstrated increases over time in global cortical and regional [11C] PIB DVR [98]. The results indicate that overall magnitude of change may be dependent on the duration of follow-up; with smaller changes resulting from shorter follow-up. A low average rate of Aβ growth in a group may be also due to individual variability, as some individuals demonstrate substantial increases in Aβ accumulation over time, while others, especially older individuals, may show no increases or even decreases in [11C] PIB uptake.
Villemagne et al. [95] recently reported significant increases in mean cortical [11C] PIB SUVR after 20-month follow up in DAT but not in mild cognitive impairment or CN individuals. However CN participants demonstrated more substantial increase after 38-month follow-up compared to individuals with mild cognitive impairment and DAT, and only CN individuals demonstrated significant increase in mean cortical and regional (orbitofrontal and dorsolateral prefrontal cortex) [11C] PIB SUVR between 20 and 38 months of follow-up [95]. Objective cognitive impairment was demonstrated in 5 of 32 CN individuals with elevated baseline [11C] PIB SUVR at 20-month follow-up and in 8 of 10 individuals who reached the 38-month time point. These 8 individuals who demonstrated cognitive impairment had significantly lower memory scores, higher baseline [11C] PIB SUVR, and higher [11C] PIB SUVR increases than those individuals who did not progress. These data suggest initial rise and then plateau in DAT individuals, but continuously progressive Aβ accumulation with clinical worsening in CN individuals with high Aβ burden.
Increasing age and genetic background are the strongest known risk factors for AD. The APOE ε4 allele is the major genetic susceptibility factor for late-onset AD, with an expressing gene dose-dependent risk for the development of DAT at an earlier age of onset [133–135]. Other isoforms of APOE are considered to be neutral (APOE ε3) or even protective (APOE ε2) for AD risk [136, 137]. The increased risk of APOE ε4 for AD may be mediated by disturbances in cerebral Aβ metabolism [138–140]. There is isoform-dependent propensity (ε4 > ε3 > ε2) for Aβ deposition in experimental animals [141, 142], and in humans [96]. APOE ε4 carriers have increased cerebral amyloid deposition compared to non-carriers [143–145]. The role of APOE ε4 in promoting AD appears to be directly related to its effect on AD pathology, because its association with clinically diagnosed DAT is non-significant after controlling for the densities of senile plaques and neurofibrillary tangles in autopsied individuals [146]. Consistent with this premise, an APOE genotype effect on Aβ load has been demonstrated in individuals with moderate DAT [147] and in CN individuals [96, 124, 126, 148, 149]. In a large cohort of CN individuals, APOE ε4 demonstrated a powerful dose-dependent effect on cerebral Aβ deposition as measured with [11C] PIB and on CSF levels of Aβ42, but not tau or ptau181 levels [96]. These data suggest that Aβ abnormalities, but not tau abnormalities, initiate the pathological cascade of preclinical AD.
Increasing evidence suggests that preclinical AD is not benign but eventually produces sufficient synaptic and neuronal damage to cause cognitive decline and other symptoms of AD [36, 150]. Reduced levels of CSF Aβ42 in CN older adults are associated with whole brain atrophy [151], and with hypometabolism in the medial temporal lobe [152]. CN older individuals with elevated [11C]PIB binding levels also demonstrate multiregional brain atrophy [153–155], cerebral cortical thinning [156], aberrant default network activity and functional MRI connectivity deficits similar to AD [157–160], decreased task-induced fMRI deactivation in the default network regions [161], lower performance on a demanding test of associative memory retrieval [162], episodic memory deficits [123, 163], as well as longitudinal cognitive decline [153]. CN individuals with elevated MCBP are at significantly greater risk of developing the symptomatic stages of AD than individuals with less or no [11C]PIB retention [150]. Moreover, this [11C]PIB predictive effect is restricted to symptomatic AD and does not encompass non-DAT causes of mild dementia [150]. Villemagne et al [95] reported that there is a 16% risk of developing mild cognitive impairment or DAT over 20 months and 25% risk by 3 years in CN individuals with high [11C]PIB retention. Higher educational attainment [119] or socioeconomic status [164] may permit individuals with preclinical AD, as ascertained by [11C]PIB, to better tolerate AD pathology without obvious cognitive deterioration, suggesting that they have a greater reserve against the clinical expression of AD [165].
Recently it has been suggested that certain lifestyle practices such as physical exercise could potentially deter or slow disease progression [166]. Physical exercise has been recognized as preserving not only cardiovascular but also brain and cognitive health in older adults [167]. It has been recommended by Alzheimer’s Association to clinicians as a way to maintain cognitive functioning in AD and enhance a patient’s quality of life, and studies suggested that exercise may reduce risk of cognitive decline and dementia [168, 169], and may have beneficial effects on AD-related pathology [170–173] including Aβ deposition as measured with [11C]PIB PET [166].
Hinrichs et al. [97] recently studied the heritability of Aβ deposition as expressed by MCBP using [11C]PIB PET and demonstrated that MCBP is a genetic trait with significant unique variability and that other Aβ related traits such as cerebrospinal fluid Aβ42 or APOE ε4 genotype do not fully explain the variance in MCBP. The predictive accuracy of MCBP alone for identification of symptomatic AD is quite high but it can be improved substantially when MCBP is used together with other factors including education, normalized whole brain volume, physical health rating, gender and use of medications that may interfere with cognition [174].
Research and therapeutic directions in preclinical AD
Further directions include the need in carefully organized longitudinal studies with multiple repeated imaging and other diagnostic assessments. While substantial data has been collected in AD using anatomical, metabolic and molecular imaging techniques, most data is cross-sectional and those few longitudinal studies are limited in number of participants and do not have enough temporal resolution to characterize the onset and growth of Aβ plaques. More longitudinal data is needed to demonstrate whether Aβ plaque levels stabilize very quickly after appearing, or alternatively, there is a slow and steady accumulation of Aβ plaques over a prolonged period of time. Longitudinal studies are critical also for the development of a reliable tool for documentation of the transfer from healthy aging to preclinical AD. This conversion may be determined by some threshold level of global Aβ deposition, but this arbitrarily approach should be evaluated in larger samples and validated by clinicopathological correlations to better characterize the incidence of preclinical AD. Time of conversion is very likely the most important period for the selection for preventive treatment trials.
It should be noted that careful design of clinical trials to test disease-modifying agents is critical, with largely enough sample sizes and optimal parameters chosen to adequately power these trials. Government and industry officials as well as academia researchers should consider the optimum use of the clinical trials design for disease-modifying agents on AD in their effort to search for the treatments with the potential to modify the underlying pathophysiology of AD [175]. Starting as early as possible before the appearance of cognitive symptoms, the preclinical stage of AD should be a critical strategy for preventive therapies aimed on decreasing production, increasing clearance, decreasing aggregation and removing aggregates of Aβ. It is likely that the metabolic changes in Aβ-prone regions may actually begin at the same time as observed plaque development in CN subjects, suggesting neuronal dysfunction is a very early part of the pathological cascade and there would be an incentive to begin treatment early as well.
Combining the efforts of multiple institutions and creating joint databases of clinical and imaging information is of critical importance for successful research and clinical efforts. A good example is the Alzheimer’s Disease Neuroimaging Initiative (ADNI), a multicenter research project that studies changes of cognition, brain structure and function, and biomarkers in elderly healthy individuals, participants with mild cognitive impairment and persons with symptomatic AD with a major goal to determine and validate MRI, PET and CSF/blood biomarkers as predictors and outcomes for use in clinical trials of AD treatments[176–183].
Another good example is related to autosomal dominant AD, which, representing less than 1% of all AD cases, may nevertheless be considered as one of the best populations for early preventive trials because it allows treating asymptomatic young adults, not affected by other brain diseases seen commonly in older individuals, and years or decades before clinical onset [184]. Due to the geographically dispersed nature of autosomal dominant families and relative rarity of the disease, an international network of research centers, known as Dominantly Inherited Alzheimer’s Network (DIAN) has been established to enable adequately powered longitudinal multicenter studies and clinical trials in this unique and highly informative population [184].
Highlights.
Recent advances PET imaging now make it possible to detect AD amyloid pathology in the preclinical stagse of the disease, in cognitively normal (CN) individuals. This review focuses on amyloid PET imaging for AD, including the biological basis of the imaging agents, data
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 2.Holtzman DM, Morris JC, Goate AM. Alzheimer’s disease: the challenge of the second century. Sci Transl Med. 2011;3 doi: 10.1126/scitranslmed.3002369. 77sr71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuchibhotla KV, Goldman ST, Lattarulo CR, Wu HY, Hyman BT, Bacskai BJ. Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks. Neuron. 2008;59:214–225. doi: 10.1016/j.neuron.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann.Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 5.Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Arch Neurol. 2001;58:1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 6.Walton HS, Dodd PR. Glutamate-glutamine cycling in Alzheimer’s disease. Neurochem Int. 2007;50:1052–1066. doi: 10.1016/j.neuint.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 7.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology. 1991;41:479–486. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- 8.Dickson TC, Vickers JC. The morphological phenotype of beta-amyloid plaques and associated neuritic changes in Alzheimer’s disease. Neuroscience. 2001;105:99–107. doi: 10.1016/s0306-4522(01)00169-5. [DOI] [PubMed] [Google Scholar]
- 9.Kuo YM, Emmerling MR, Vigo-Pelfrey C, Kasunic TC, Kirkpatrick JB, Murdoch GH, Ball MJ, Roher AE. Water-soluble Abeta (N-40, N-42) oligomers in normal and Alzheimer disease brains. J Biol Chem. 1996;271:4077–4081. doi: 10.1074/jbc.271.8.4077. [DOI] [PubMed] [Google Scholar]
- 10.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson-Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 11.Walker LC, Ibegbu CC, Todd CW, Robinson HL, Jucker M, LeVine H, 3rd, Gandy S. Emerging prospects for the disease-modifying treatment of Alzheimer’s disease. Biochem Pharmacol. 2005;69:1001–1008. doi: 10.1016/j.bcp.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer I, Boada Rovira M, Sanchez Guerra ML, Rey MJ, Costa-Jussa F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004;14:11–20. doi: 10.1111/j.1750-3639.2004.tb00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, Seubert P, Games D, Kirby L, Schenk D. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology. 2005;64:129–131. doi: 10.1212/01.WNL.0000148590.39911.DF. [DOI] [PubMed] [Google Scholar]
- 14.Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, Weller RO. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: a case report. Nat Med. 2003;9:448–452. doi: 10.1038/nm840. [DOI] [PubMed] [Google Scholar]
- 15.Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. doi: 10.1016/0896-6273(94)90458-8. [DOI] [PubMed] [Google Scholar]
- 16.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 17.Scheuner D, Eckman C, Jensen M, Song X, Citron M, Suzuki N, Bird TD, Hardy J, Hutton M, Kukull W, Larson E, Levy-Lahad E, Viitanen M, Peskind E, Poorkaj P, Schellenberg G, Tanzi R, Wasco W, Lannfelt L, Selkoe D, Younkin S. Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer’s disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer’s disease. Nat Med. 1996;2:864–870. doi: 10.1038/nm0896-864. [DOI] [PubMed] [Google Scholar]
- 18.Mawuenyega KG, Sigurdson W, Ovod V, Munsell L, Kasten T, Morris JC, Yarasheski KE, Bateman RJ. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardy J, Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-v. [DOI] [PubMed] [Google Scholar]
- 20.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 21.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 22.Small SA, Duff K. Linking Abeta and tau in late-onset Alzheimer's disease: a dual pathway hypothesis. Neuron. 2008;60:534–542. doi: 10.1016/j.neuron.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer's disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hyman BT. Amyloid-Dependent and Amyloid-Independent Stages of Alzheimer Disease. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.70. [DOI] [PubMed] [Google Scholar]
- 25.Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau. Nat Neurosci. 1998;1:355–358. doi: 10.1038/1565. [DOI] [PubMed] [Google Scholar]
- 26.Price DL, Sisodia SS. Mutant genes in familial Alzheimer's disease and transgenic models. Annu Rev Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 27.Tanzi RE, Kovacs DM, Kim TW, Moir RD, Guenette SY, Wasco W. The gene defects responsible for familial Alzheimer's disease. Neurobiol Dis. 1996;3:159–168. doi: 10.1006/nbdi.1996.0016. [DOI] [PubMed] [Google Scholar]
- 28.Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, Yu CE, Jondro PD, Schmidt SD, Wang K, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995;269:973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- 29.Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, Chi H, Lin C, Li G, Holman K, Tsuda T, Mar L, Foncin JF, Bruni AC, Montesi MP, Sorbi S, Rainero I, Pinessi L, Nee L, Chumakov I, Pollen D, Brookes A, Sanseau P, Polinsky RJ, Wasco W, Da Silva HA, Haines JL, Perkicak-Vance MA, Tanzi RE, Roses AD, Fraser PE, Rommens JM, St George-Hyslop PH. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 30.Kauwe JS, Jacquart S, Chakraverty S, Wang J, Mayo K, Fagan AM, Holtzman DM, Morris JC, Goate AM. Extreme cerebrospinal fluid amyloid beta levels identify family with late-onset Alzheimer's disease presenilin 1 mutation. Ann Neurol. 2007;61:446–453. doi: 10.1002/ana.21099. [DOI] [PubMed] [Google Scholar]
- 31.Selkoe DJ. Soluble oligomers of the amyloid beta-protein impair synaptic plasticity and behavior. Behav Brain Res. 2008;192:106–113. doi: 10.1016/j.bbr.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cirrito JR, Yamada KA, Finn MB, Sloviter RS, Bales KR, May PC, Schoepp DD, Paul SM, Mennerick S, Holtzman DM. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005;48:913–922. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Marcello E, Epis R, Di Luca M. Amyloid flirting with synaptic failure: towards a comprehensive view of Alzheimer's disease pathogenesis. Eur J Pharmacol. 2008;585:109–118. doi: 10.1016/j.ejphar.2007.11.083. [DOI] [PubMed] [Google Scholar]
- 34.Vlassenko AG, Vaishnavi SN, Couture L, Sacco D, Shannon BJ, Mach RH, Morris JC, Raichle ME, Mintun MA. Spatial correlation between brain aerobic glycolysis and amyloid-beta (Abeta ) deposition. Proc Natl Acad Sci U S A. 2010;107:17763–17767. doi: 10.1073/pnas.1010461107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vaishnavi SN, Vlassenko AG, Rundle MM, Snyder AZ, Mintun MA, Raichle ME. Regional aerobic glycolysis in the human brain. Proc Natl Acad Sci U S A. 2010;107:17757–17762. doi: 10.1073/pnas.1010459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris JC, Price AL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer's disease. J.Mol.Neurosci. 2001;17:101–118. doi: 10.1385/jmn:17:2:101. [DOI] [PubMed] [Google Scholar]
- 37.Shim YS, Morris JC. Biomarkers predicting Alzheimer's disease in cognitively normal aging. J Clin Neurol. 7:60–68. doi: 10.3988/jcn.2011.7.2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ewers M, Sperling RA, Klunk WE, Weiner MW, Hampel H. Neuroimaging markers for the prediction and early diagnosis of Alzheimer's disease dementia. Trends Neurosci. 2011;34:430–442. doi: 10.1016/j.tins.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR, Jr, Kaye J, Montine TJ, Park DC, Reiman EM, Rowe CC, Siemers E, Stern Y, Yaffe K, Carrillo MC, Thies B, Morrison-Bogorad M, Wagster MV, Phelps CH. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jack CR, Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9:119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blennow K, Vanmechelen E, Hampel H. CSF total tau, Abeta42 and phosphorylated tau protein as biomarkers for Alzheimer's disease. Mol Neurobiol. 2001;24:87–97. doi: 10.1385/MN:24:1-3:087. [DOI] [PubMed] [Google Scholar]
- 42.Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, Larossa GN, Spinner ML, Klunk WE, Mathis CA, DeKosky ST, Morris JC, Holtzman DM. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann.Neurol. 2006;59:512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 43.Fagan AM, Mintun MA, Shah AR, Aldea P, Roe CM, Mach RH, Marcus D, Morris JC, Holtzman DM. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med. 2009;1:371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Riemenschneider M, Wagenpfeil S, Diehl J, Lautenschlager N, Theml T, Heldmann B, Drzezga A, Jahn T, Forstl H, Kurz A. Tau and Abeta42 protein in CSF of patients with frontotemporal degeneration. Neurology. 2002;58:1622–1628. doi: 10.1212/wnl.58.11.1622. [DOI] [PubMed] [Google Scholar]
- 45.Sjogren M, Minthon L, Davidsson P, Granerus AK, Clarberg A, Vanderstichele H, Vanmechelen E, Wallin A, Blennow K. CSF levels of tau, beta-amyloid(1–42) and GAP-43 in frontotemporal dementia, other types of dementia and normal aging. J Neural Transm. 2000;107:563–579. doi: 10.1007/s007020070079. [DOI] [PubMed] [Google Scholar]
- 46.Clark CM, Xie S, Chittams J, Ewbank D, Peskind E, Galasko D, Morris JC, McKeel DW, Jr, Farlow M, Weitlauf SL, Quinn J, Kaye J, Knopman D, Arai H, Doody RS, DeCarli C, Leight S, Lee VM, Trojanowski JQ. Cerebrospinal fluid tau and beta-amyloid: how well do these biomarkers reflect autopsy-confirmed dementia diagnoses? Arch Neurol. 2003;60:1696–1702. doi: 10.1001/archneur.60.12.1696. [DOI] [PubMed] [Google Scholar]
- 47.Bateman RJ, Wen G, Morris JC, Holtzman DM. Fluctuations of CSF amyloid-beta levels: implications for a diagnostic and therapeutic biomarker. Neurology. 2007;68:666–669. doi: 10.1212/01.wnl.0000256043.50901.e3. [DOI] [PubMed] [Google Scholar]
- 48.Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM. Decreased beta-amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 49.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 50.Shoji M, Matsubara E, Kanai M, Watanabe M, Nakamura T, Tomidokoro Y, Shizuka M, Wakabayashi K, Igeta Y, Ikeda Y, Mizushima K, Amari M, Ishiguro K, Kawarabayashi T, Harigaya Y, Okamoto K, Hirai S. Combination assay of CSF tau, A beta 1–40 and A beta 1–42(43) as a biochemical marker of Alzheimer's disease. J Neurol Sci. 1998;158:134–140. doi: 10.1016/s0022-510x(98)00122-1. [DOI] [PubMed] [Google Scholar]
- 51.Itoh N, Arai H, Urakami K, Ishiguro K, Ohno H, Hampel H, Buerger K, Wiltfang J, Otto M, Kretzschmar H, Moeller HJ, Imagawa M, Kohno H, Nakashima K, Kuzuhara S, Sasaki H, Imahori K. Large-scale, multicenter study of cerebrospinal fluid tau protein phosphorylated at serine 199 for the antemortem diagnosis of Alzheimer's disease. Ann Neurol. 2001;50:150–156. doi: 10.1002/ana.1054. [DOI] [PubMed] [Google Scholar]
- 52.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. erebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 53.Shaw LM, Vanderstichele H, Knapik-Czajka M, Clark CM, Aisen PS, etersen RC, Blennow K, Soares H, Simon A, Lewczuk P, Dean R, Siemers E, Potter W, Lee VM, Trojanowski JQ. Cerebrospinal fluid biomarker signature in Alzheimer's disease neuroimaging initiative subjects. Ann Neurol. 2009;65:403–413. doi: 10.1002/ana.21610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, Sjoegren M, DeBernardis J, Kerkman D, Ishiguro K, Ohno H, Vanmechelen E, Vanderstichele H, McCulloch C, Moller HJ, Davies P, Blennow K. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 55.Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttila T, Schapiro MB, Rapoport SI, Moller HJ, Davies P, Hampel H. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 56.Li G, Sokal I, Quinn JF, Leverenz JB, Brodey M, Schellenberg GD, Kaye JA, Raskind A, Zhang J, Peskind ER, Montine TJ. CSF tau/Abeta42 ratio for increased risk of mild cognitive impairment: a follow-up study. Neurology. 2007;69:631–639. doi: 10.1212/01.wnl.0000267428.62582.aa. [DOI] [PubMed] [Google Scholar]
- 57.Klunk WE, Mathis CA. The future of amyloid-beta imaging: a tale of radionuclides and tracer proliferation. Curr Opin Neurol. 2008;21:683–687. doi: 10.1097/WCO.0b013e3283168e1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vallabhajosula S. Positron emission tomography radiopharmaceuticals for imaging brain Beta-amyloid. Semin Nucl Med. 2011;41:283–299. doi: 10.1053/j.semnuclmed.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 59.Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol. 2009;21:117–128. doi: 10.3233/BEN-2009-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Agdeppa ED, Kepe V, Liu J, Flores-Torres S, Satyamurthy N, Petric A, Cole GM, Small GW, Huang SC, Barrio JR. Binding characteristics of radiofluorinated 6-dialkylamino-2-naphthylethylidene derivatives as positron emission tomography imaging probes for beta-amyloid plaques in Alzheimer's disease. J.Neurosci. 2001;21:RC189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang SC, Barrio JR. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am.J.Geriatr.Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]
- 62.Noda A, Murakami Y, Nishiyama S, Fukumoto D, Miyoshi S, Tsukada H, Nishimura S. Amyloid imaging in aged and young macaques with [11C]PIB and [18F]FDDNP. Synapse. 2008;62:472–475. doi: 10.1002/syn.20508. [DOI] [PubMed] [Google Scholar]
- 63.Small GW, Kepe V, Ercoli LM, Siddarth P, Bookheimer SY, Miller KJ, Lavretsky H, Burggren AC, Cole GM, Vinters HV, Thompson PM, Huang SC, Satyamurthy N, Phelps ME, Barrio JR. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 64.Thompson PW, Ye L, Morgenstern JL, Sue L, Beach TG, Judd DJ, Shipley NJ, Libri V, Lockhart A. Interaction of the amyloid imaging tracer FDDNP with hallmark Alzheimer's disease pathologies. J Neurochem. 2009;109:623–630. doi: 10.1111/j.1471-4159.2009.05996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ono M, Wilson A, Nobrega J, Westaway D, Verhoeff P, Zhuang ZP, Kung MP, Kung HF. 11C–labeled stilbene derivatives as Abeta-aggregate-specific PET imaging agents for Alzheimer's disease. Nucl Med Biol. 2003;30:565–571. doi: 10.1016/s0969-8051(03)00049-0. [DOI] [PubMed] [Google Scholar]
- 66.Verhoef N, Wilson AA, Takeshita S, Trop L, Hussey D, Singh K, Kung HF, Houle S. In vivo imaging of Alzheimer disease á-amyloid with [ 1 1 C]SB-13 PET. Am.J.Geriatr.Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 67.Rowe CC, Ackerman U, Browne W, Mulligan R, Pike KL, O’Keefe G, Tochon-Danguy H, Chan G, Berlangieri SU, Jones G, Dickinson-Rowe KL, Kung HP, Zhang W, Kung MP, Skovronsky D, Dyrks T, Holl G, Krause S, Friebe M, Lehman L, Lindemann S, Dinkelborg LM, Masters CL, Villemagne VL. Imaging of amyloid beta in Alzheimer's disease with 18F–BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 68.Koole M, Lewis DM, Buckley C, Nelissen N, Vandenbulcke M, Brooks DJ, Vandenberghe R, Van Laere K. Whole-body biodistribution and radiation dosimetry of 18F–GE067: a radioligand for in vivo brain amyloid imaging. J Nucl Med. 2009;50:818–822. doi: 10.2967/jnumed.108.060756. [DOI] [PubMed] [Google Scholar]
- 69.Kung HF, Choi SR, Qu W, Zhang W, Skovronsky D. 18F stilbenes and styrylpyridines for PET imaging of A beta plaques in Alzheimer's disease: a miniperspective. J Med Chem. 2010;53:933–941. doi: 10.1021/jm901039z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang W, Kung MP, Oya S, Hou C, Kung HF. 18F–labeled styrylpyridines as PET agents for amyloid plaque imaging. Nucl Med Biol. 2007;34:89–97. doi: 10.1016/j.nucmedbio.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 71.Choi SR, Golding G, Zhuang Z, Zhang W, Lim N, Hefti F, Benedum TE, Kilbourn MR, Skovronsky D, Kung HF. Preclinical properties of 18F–AV-45: a PET agent for Abeta plaques in the brain. J Nucl Med. 2009;50:1887–1894. doi: 10.2967/jnumed.109.065284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Liu Y, Zhu L, Plossl K, Choi SR, Qiao H, Sun X, Li S, Zha Z, Kung HF. Optimization of automated radiosynthesis of [18F]AV-45: a new PET imaging agent for Alzheimer's disease. Nucl Med Biol. 2010;37:917–925. doi: 10.1016/j.nucmedbio.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wong DF, Rosenberg PB, Zhou Y, Kumar A, Raymont V, Ravert HT, Dannals RF, Nandi A, Brasic JR, Ye W, Hilton J, Lyketsos C, Kung HF, Joshi AD, Skovronsky DM, Pontecorvo MJ. In vivo imaging of amyloid deposition in Alzheimer disease using the radioligand 18F–AV-45 (florbetapir [corrected] F 18) J Nucl Med. 2010;51:913–920. doi: 10.2967/jnumed.109.069088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin KJ, Hsu WC, Hsiao IT, Wey SP, Jin LW, Skovronsky D, Wai YY, Chang HP, Lo CW, Yao CH, Yen TC, Kung MP. Whole-body biodistribution and brain PET imaging with [18F]AV-45, a novel amyloid imaging agent--a pilot study. Nucl Med Biol. 2010;37:497–508. doi: 10.1016/j.nucmedbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 75.Clark CM, Schneider JA, Bedell BJ, Beach TG, Bilker WB, Mintun MA, Pontecorvo MJ, Hefti F, Carpenter AP, Flitter ML, Krautkramer MJ, Kung HF, Coleman RE, Doraiswamy PM, Fleisher AS, Sabbagh MN, Sadowsky CH, Reiman PE, Zehntner SP, Skovronsky DM. Use of florbetapir-PET for imaging beta-amyloid pathology. JAMA. 2011;305:275–283. doi: 10.1001/jama.2010.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kung MP, Hou C, Zhuang ZP, Zhang B, Skovronsky D, Trojanowski JQ, Lee VM, Kung HF. IMPY: an improved thioflavin-T derivative for in vivo labeling of beta-amyloid plaques. Brain Res. 2002;956:202–210. doi: 10.1016/s0006-8993(02)03436-4. [DOI] [PubMed] [Google Scholar]
- 77.Zhuang ZP, Kung MP, Wilson A, Lee CW, Plossl K, Hou C, Holtzman DM, Kung HF. Structure-activity relationship of imidazo[1,2-a]pyridines as ligands for detecting beta-amyloid plaques in the brain. J.Med.Chem. 2003;46:237–243. doi: 10.1021/jm020351j. [DOI] [PubMed] [Google Scholar]
- 78.Newberg AB, Wintering NA, Plossl K, Hochold J, Stabin MG, Watson M, Skovronsky D, Clark CM, Kung MP, Kung HF. Safety, biodistribution, and dosimetry of 123I–IMPY: a novel amyloid plaque-imaging agent for the diagnosis of Alzheimer's disease. J Nucl Med. 2006;47:748–754. [PubMed] [Google Scholar]
- 79.Maya Y, Ono M, Watanabe H, Haratake M, Saji H, Nakayama M. Novel radioiodinated aurones as probes for SPECT imaging of beta-amyloid plaques in the brain. Bioconjug Chem. 2009;20:95–101. doi: 10.1021/bc8003292. [DOI] [PubMed] [Google Scholar]
- 80.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann.Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 81.Nordberg A. PET imaging of amyloid in Alzheimer's disease. Lancet Neurol. 2004;3:519–527. doi: 10.1016/S1474-4422(04)00853-1. [DOI] [PubMed] [Google Scholar]
- 82.Engler H, Forsberg A, Almkvist O, Blomquist G, Larsson E, Savitcheva I, Wall A, Ringheim A, Langstrom B, Nordberg A. Two-year follow-up of amyloid deposition in patients with Alzheimer's disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 83.Drzezga A, Grimmer T, Henriksen G, Stangier I, Perneczky R, Diehl-Schmid J, Mathis CA, Klunk WE, Price J, DeKosky S, Wester HJ, Schwaiger M, Kurz A. Imaging of amyloid plaques and cerebral glucose metabolism in semantic dementia and Alzheimer's disease. Neuroimage. 2008;39:619–633. doi: 10.1016/j.neuroimage.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 84.Mintun MA, Larossa GN, Sheline YI, Dence CS, Lee SY, Mach RH, Klunk WE, Mathis CA, DeKosky ST, Morris JC. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006;67:446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 85.Lopresti BJ, Klunk WE, Mathis CA, Hoge JA, Ziolko SK, Lu X, Meltzer CC, Schimmel K, Tsopelas ND, DeKosky ST, Price JC. Simplified Quantification of Pittsburgh Compound B Amyloid Imaging PET Studies: A Comparative Analysis. J.Nucl.Med. 2005;46:1959–1972. [PubMed] [Google Scholar]
- 86.Mathis CA, Wang Y, Holt DP, Huang GF, Debnath ML, Klunk WE. Synthesis and evaluation of 11C–labeled 6-substituted 2-arylbenzothiazoles as amyloid imaging agents. J.Med.Chem. 2003;46:2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- 87.Price JC, Klunk WE, Lopresti BJ, Lu X, Hoge JA, Ziolko SK, Holt DP, Meltzer CC, DeKosky ST, Mathis CA. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J.Cereb.Blood Flow Metab. 2005;25:1528–1547. doi: 10.1038/sj.jcbfm.9600146. [DOI] [PubMed] [Google Scholar]
- 88.O’Keefe GJ, Saunder TH, Ng S, Ackerman U, Tochon-Danguy HJ, Chan JG, Gong S, Dyrks T, Lindemann S, Holl G, Dinkelborg L, Villemagne V, Rowe CC. Radiation dosimetry of beta-amyloid tracers 11C–PiB and 18F–BAY94-9172. J Nucl Med. 2009;50:309–315. doi: 10.2967/jnumed.108.055756. [DOI] [PubMed] [Google Scholar]
- 89.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J.Cereb.Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 90.Mikhno A, Devanand D, Pelton G, Cuasay K, Gunn R, Upton N, Lai RY, Libri V, Mann JJ, Parsey RV. Voxel-based analysis of 11C–PIB scans for diagnosing Alzheimer's disease. J Nucl Med. 2008;49:1262–1269. doi: 10.2967/jnumed.107.049932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Raniga P, Bourgeat P, Fripp J, Acosta O, Villemagne VL, Rowe C, Masters CL, Jones G, O’Keefe G, Salvado O, Ourselin S. Automated (11)C-PiB standardized uptake value ratio. Acad Radiol. 2008;15:1376–1389. doi: 10.1016/j.acra.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 92.McNamee RL, Yee SH, Price JC, Klunk WE, Rosario B, Weissfeld L, Ziolko S, Berginc M, Lopresti B, Dekosky S, Mathis CA. Consideration of optimal time window for Pittsburgh compound B PET summed uptake measurements. J Nucl Med. 2009;50:348–355. doi: 10.2967/jnumed.108.057612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mourik JE, Lubberink M, Schuitemaker A, Tolboom N, van Berckel BN, Lammertsma AA, Boellaard R. Image-derived input functions for PET brain studies. Eur J Nucl Med Mol Imaging. 2009;36:463–471. doi: 10.1007/s00259-008-0986-8. [DOI] [PubMed] [Google Scholar]
- 94.Aizenstein HJ, Nebes RD, Saxton JA, Price JC, Mathis CA, Tsopelas ND, Ziolko SK, James JA, Snitz BE, Houck PR, Bi W, Cohen AD, Lopresti BJ, DeKosky ST, Halligan EM, Klunk WE. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol. 2008;65:1509–1517. doi: 10.1001/archneur.65.11.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Villemagne VL, Pike KE, Chetelat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O’Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC. Longitudinal assessment of Abeta and cognition in aging and Alzheimer disease. Ann Neurol. 2011;69:181–192. doi: 10.1002/ana.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Morris JC, Roe CM, Xiong C, Fagan AM, Goate AM, Holtzman DM, Mintun MA. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol. 2010;67:122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hinrichs AL, Mintun MA, Head D, Fagan AM, Holtzman DM, Morris JC, Goate AM. Cortical binding of pittsburgh compound B, an endophenotype for genetic studies of Alzheimer's disease. Biol Psychiatry. 2010;67:581–583. doi: 10.1016/j.biopsych.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sojkova J, Zhou Y, An Y, Kraut MA, Ferrucci L, Wong DF, Resnick SM. Longitudinal Patterns of {beta}-Amyloid Deposition in Nondemented Older Adults. Arch Neurol. 2011;68:644–649. doi: 10.1001/archneurol.2011.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 100.Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- 101.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. Anautomated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 102.Lehmann M, Rohrer JD, Clarkson MJ, Ridgway GR, Scahill RI, Modat M, Warren JD, Ourselin S, Barnes J, Rossor MN, Fox NC. Reduced cortical thickness in the posterior cingulate gyrus is characteristic of both typical and atypical Alzheimer's disease. J Alzheimers Dis. 2010;20:587–598. doi: 10.3233/JAD-2010-1401. [DOI] [PubMed] [Google Scholar]
- 103.Mueller SG, Schuff N, Yaffe K, Madison C, Miller B, Weiner MW. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer's disease. Hum Brain Mapp. 2010;31:1339–1347. doi: 10.1002/hbm.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fortea J, Sala-Llonch R, Bartres-Faz D, Llado A, Sole-Padulles C, Bosch B, Antonell A, Olives J, Sanchez-Valle R, Molinuevo JL, Rami L. Cognitively preserved subjects with transitional cerebrospinal fluid ss-amyloid 1–42 values have thicker cortex in Alzheimer's disease vulnerable areas. Biol Psychiatry. 70:183–190. doi: 10.1016/j.biopsych.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 105.Becker JA, Hedden T, Carmasin J, Maye J, Rentz DM, Putcha D, Fischl B, Greve DN, Marshall GA, Salloway S, Marks D, Buckner RL, Sperling RA, Johnson KA. Amyloid-beta associated cortical thinning in clinically normal elderly. Ann Neurol. 2011;69:1032–1042. doi: 10.1002/ana.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Seibert TM, Brewer JB. Default network correlations analyzed on native surfaces. J Neurosci Methods. 198:301–311. doi: 10.1016/j.jneumeth.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Su Y, Vlassenko A, Benzinger T, D’Angelo G, Blazey T, Vora S, Morris JC, Mintun M. FreeSurfer regional analysis of beta-amyloid deposition with [11C]PIB positron emission tomography. Alzheimers Dement. 2011;7:S227. [Google Scholar]
- 108.Ikonomovic MD, Klunk WE, Abrahamson EE, Mathis CA, Price JC, Tsopelas ND, Lopresti BJ, Ziolko S, Bi W, Paljug WR, Debnath ML, Hope CE, Isanski BA, Hamilton RL, DeKosky ST. Post-mortem correlates of in vivo PiB-PET amyloid imaging in a typical case of Alzheimer's disease. Brain. 2008;131:1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bacskai BJ, Frosch MP, Freeman SH, Raymond SB, Augustinack JC, Johnson KA, Irizarry MC, Klunk WE, Mathis CA, Dekosky ST, Greenberg SM, Hyman BT, Growdon JH. Molecular imaging with Pittsburgh Compound B confirmed at autopsy: a case report. Arch Neurol. 2007;64:431–434. doi: 10.1001/archneur.64.3.431. [DOI] [PubMed] [Google Scholar]
- 110.Leinonen V, Alafuzoff I, Aalto S, Suotunen T, Savolainen S, Nagren K, Tapiola T, Pirttila T, Rinne J, Jaaskelainen JE, Soininen H, Rinne JO. Assessment of beta-amyloid in a frontal cortical brain biopsy specimen and by positron emission tomography with carbon 11-labeled Pittsburgh Compound B. Arch Neurol. 2008;65:1304–1309. doi: 10.1001/archneur.65.10.noc80013. [DOI] [PubMed] [Google Scholar]
- 111.Svedberg MM, Hall H, Hellstrom-Lindahl E, Estrada S, Guan Z, Nordberg A, Langstrom B. [(11)C]PIB-amyloid binding and levels of Abeta40 and Abeta42 in postmortem brain tissue from Alzheimer patients. Neurochem Int. 2009;54:347–357. doi: 10.1016/j.neuint.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 112.Klunk WE, Wang Y, Huang GF, Debnath ML, Holt DP, Shao L, Hamilton RL, Ikonomovic MD, DeKosky ST, Mathis CA. The binding of 2-(4’-methylaminophenyl)benzothiazole to postmortem brain homogenates is dominated by the amyloid component. J.Neurosci. 2003;23:2086–2092. doi: 10.1523/JNEUROSCI.23-06-02086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lockhart A, Lamb JR, Osredkar T, Sue LI, Joyce JN, Ye L, Libri V, Leppert D, Beach TG. PIB is a non-specific imaging marker of amyloid-beta (Abeta) peptide-related cerebral amyloidosis. Brain. 2007;130:2607–2615. doi: 10.1093/brain/awm191. [DOI] [PubMed] [Google Scholar]
- 114.Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, Smith EE, Rosand J, Rentz DM, Klunk WE, Mathis CA, Price JC, Dekosky ST, Fischman AJ, Greenberg SM. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 115.Mathis C, Lopresti B, Mason N, Price J, Flatt N, Bi W, Ziolko S, DeKosky S, Klunk W. Comparison of the amyloid imaging agents [F-18]3’-F-PIB and [C-11]PIB in Alzheimer's disease and control subjects. J Nucl Med 48. 2007 Supplement 2:56. [Google Scholar]
- 116.Serdons K, Verduyckt T, Vanderghinste D, Cleynhens J, Borghgraef P, Vermaelen P, Terwinghe C, Van Leuven F, Van Laere K, Kung H, Bormans G, Verbruggen A. Synthesis of 18F–labelled 2-(4’-fluorophenyl)-1,3-benzothiazole and evaluation as amyloid imaging agent in comparison with [11C]PIB. Bioorg Med Chem Lett. 2009;19:602–605. doi: 10.1016/j.bmcl.2008.12.069. [DOI] [PubMed] [Google Scholar]
- 117.Stern Y. Cognitive reserve and Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20:112–117. doi: 10.1097/01.wad.0000213815.20177.19. [DOI] [PubMed] [Google Scholar]
- 118.Mortimer JA, Borenstein AR, Gosche KM, Snowdon DA. Very early detection of Alzheimer neuropathology and the role of brain reserve in modifying its clinical expression. J Geriatr Psychiatry Neurol. 2005;18:218–223. doi: 10.1177/0891988705281869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Roe CM, Mintun MA, D’Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol. 2008;65:1467–1471. doi: 10.1001/archneur.65.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiol Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 121.Morris JC, Storandt M, McKeel DW, Jr, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in “normal” aging: Evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 122.Galvin JE, Powlishta KK, Wilkins K, McKeel DW, Jr, Xiong C, Grant E, Storandt M, Morris JC. Predictors of preclinical Alzheimer disease and dementia: a clinicopathologic study. Arch Neurol. 2005;62:758–765. doi: 10.1001/archneur.62.5.758. [DOI] [PubMed] [Google Scholar]
- 123.Pike KE, Savage G, Villemagne VL, Ng S, Moss SA, Maruff P, Mathis CA, Klunk WE, Masters CL, Rowe CC. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain. 2007;130:2837–2844. doi: 10.1093/brain/awm238. [DOI] [PubMed] [Google Scholar]
- 124.Pike KE, Ellis KA, Villemagne VL, Good N, Chetelat G, Ames D, Szoeke C, Laws SM, Verdile G, Martins RN, Masters CL, Rowe CC. Cognition and beta-amyloid in preclinical Alzheimer's disease: Data from the AIBL study. Neuropsychologia. 2011 doi: 10.1016/j.neuropsychologia.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 125.Price JL, McKeel DW, Jr, Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC. Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging. 2009;30:1026–1036. doi: 10.1016/j.neurobiolaging.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rowe CC, Ellis KA, Rimajova M, Bourgeat P, Pike KE, Jones G, Fripp J, Tochon-Danguy H, Morandeau L, O’Keefe G, Price R, Raniga P, Robins P, Acosta O, Lenzo N, Szoeke C, Salvado O, Head R, Martins R, Masters CL, Ames D, Villemagne VL. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 127.Villemagne VL, Pike KE, Darby D, Maruff P, Savage G, Ng S, Ackermann U, Cowie TF, Currie J, Chan SG, Jones G, Tochon-Danguy H, O’Keefe G, Masters CL, Rowe CC. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia. 2008;46:1688–1697. doi: 10.1016/j.neuropsychologia.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 128.Klunk WE, Price JC, Mathis CA, Tsopelas ND, Lopresti BJ, Ziolko SK, Bi W, Hoge JA, Cohen AD, Ikonomovic MD, Saxton JA, Snitz BE, Pollen DA, Moonis M, Lippa CF, Swearer JM, Johnson KA, Rentz DM, Fischman AJ, Aizenstein HJ, DeKosky ST. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J Neurosci. 2007;27:6174–6184. doi: 10.1523/JNEUROSCI.0730-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Remes AM, Laru L, Tuominen H, Aalto S, Kemppainen N, Mononen H, Nagren K, Parkkola R, Rinne JO. Carbon 11-labeled pittsburgh compound B positron emission tomographic amyloid imaging in patients with APP locus duplication. Arch Neurol. 2008;65:540–544. doi: 10.1001/archneur.65.4.540. [DOI] [PubMed] [Google Scholar]
- 130.Villemagne VL, Ataka S, Mizuno T, Brooks WS, Wada Y, Kondo M, Jones G, Watanabe Y, Mulligan R, Nakagawa M, Miki T, Shimada H, O’Keefe GJ, Masters CL, Mori H, Rowe CC. High striatal amyloid beta-peptide deposition across different autosomal Alzheimer disease mutation types. Arch Neurol. 2009;66:1537–1544. doi: 10.1001/archneurol.2009.285. [DOI] [PubMed] [Google Scholar]
- 131.Scheinin NM, Aalto S, Koikkalainen J, Lotjonen J, Karrasch M, Kemppainen N, Viitanen M, Nagren K, Helin S, Scheinin M, Rinne JO. Follow-up of [11C]PIB uptake and brain volume in patients with Alzheimer disease and controls. Neurology. 2009;73:1186–1192. doi: 10.1212/WNL.0b013e3181bacf1b. [DOI] [PubMed] [Google Scholar]
- 132.Jack CR, Jr, Lowe VJ, Weigand SD, Wiste HJ, Senjem ML, Knopman DS, Shiung MM, Gunter JL, Boeve BF, Kemp BJ, Weiner M, Petersen RC. Serial PIB and MRI in normal, mild cognitive impairment and Alzheimer's disease: implications for sequence of pathological events in Alzheimer's disease. Brain. 2009;132:1355–1365. doi: 10.1093/brain/awp062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 134.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age sex ethnicity on the association between apolipoprotein E genotype Alzheimer A meta-analysis disease. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 135.Bertram L, Lange C, Mullin K, Parkinson M, Hsiao M, Hogan MF, Schjeide BM, Hooli B, Divito J, Ionita I, Jiang H, Laird N, Moscarillo T, Ohlsen KL, Elliott K, Wang X, Hu-Lince D, Ryder M, Murphy A, Wagner SL, Blacker D, Becker KD, Tanzi RE. Genome-wide association analysis reveals putative Alzheimer's disease susceptibility loci in addition to APOE. Am J Hum Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Corder EH, Saunders AM, Risch NJ, Strittmatter WJ, Schmechel DE, Gaskell PC, Jr, Rimmler JB, Locke PA, Conneally PM, Schmader KE, et al. Protective effect of apolipoprotein E type 2 allele for late onset Alzheimer disease. Nat Genet. 1994;7:180–184. doi: 10.1038/ng0694-180. [DOI] [PubMed] [Google Scholar]
- 137.Talbot C, Lendon C, Craddock N, Shears S, Morris JC, Goate A. Protection against Alzheimer's disease with apoE epsilon 2. Lancet. 1994;343:1432–1433. doi: 10.1016/s0140-6736(94)92557-7. [DOI] [PubMed] [Google Scholar]
- 138.Ma J, Yee A, Brewer HB, Jr, Das S, Potter H. Amyloid-associated proteins alpha 1-antichymotrypsin and apolipoprotein E promote assembly of Alzheimer beta-protein into filaments. Nature. 1994;372:92–94. doi: 10.1038/372092a0. [DOI] [PubMed] [Google Scholar]
- 139.DeMattos RB, Cirrito JR, Parsadanian M, May PC, O’Dell MA, Taylor JW, Harmony JA, Aronow BJ, Bales KR, Paul SM, Holtzman DM. ApoE and clusterin cooperatively suppress Abeta levels and deposition: evidence that ApoE regulates extracellular Abeta metabolism in vivo. Neuron. 2004;41:193–202. doi: 10.1016/s0896-6273(03)00850-x. [DOI] [PubMed] [Google Scholar]
- 140.Jiang Q, Lee CY, Mandrekar S, Wilkinson B, Cramer P, Zelcer N, Mann K, Lamb B, Willson TM, Collins JL, Richardson JC, Smith JD, Comery TA, Riddell D, Holtzman DM, Tontonoz P, Landreth GE. ApoE promotes the proteolytic degradation of Abeta. Neuron. 2008;58:681–693. doi: 10.1016/j.neuron.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Holtzman DM, Bales KR, Tenkova T, Fagan AM, Parsadanian M, Sartorius LJ, Mackey B, Olney J, McKeel D, Wozniak D, Paul SM. Apolipoprotein E isoform-dependent amyloid deposition and neuritic degeneration in a mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 2000;97:2892–2897. doi: 10.1073/pnas.050004797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fagan AM, Watson M, Parsadanian M, Bales KR, Paul SM, Holtzman DM. Human and murine ApoE markedly alters A beta metabolism before and after plaque formation in a mouse model of Alzheimer's disease. Neurobiol Dis. 2002;9:305–318. doi: 10.1006/nbdi.2002.0483. [DOI] [PubMed] [Google Scholar]
- 143.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer's disease: allelic variation and receptor interactions. Neuron. 1993;11:575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 144.Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Polvikoski T, Sulkava R, Haltia M, Kainulainen K, Vuorio A, Verkkoniemi A, Niinisto L, Halonen P, Kontula K. Apolipoprotein E, dementia, and cortical deposition of beta-amyloid protein. N Engl J Med. 1995;333:1242–1247. doi: 10.1056/NEJM199511093331902. [DOI] [PubMed] [Google Scholar]
- 146.Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Berry-Kravis E, Bienias JL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer's disease. Neurology. 2003;60:246–252. doi: 10.1212/01.wnl.0000042478.08543.f7. [DOI] [PubMed] [Google Scholar]
- 147.Drzezga A, Grimmer T, Henriksen G, Muhlau M, Perneczky R, Miederer I, Praus C, Sorg C, Wohlschlager A, Riemenschneider M, Wester HJ, Foerstl H, Schwaiger M, Kurz A. Effect of APOE genotype on amyloid plaque load and gray matter volume in Alzheimer disease. Neurology. 2009;72:1487–1494. doi: 10.1212/WNL.0b013e3181a2e8d0. [DOI] [PubMed] [Google Scholar]
- 148.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum JB, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci U S A. 2009;106:6820–6825. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Small GW, Siddarth P, Burggren AC, Kepe V, Ercoli LM, Miller KJ, Lavretsky H, Thompson PM, Cole GM, Huang SC, Phelps ME, Bookheimer SY, Barrio JR. Influence of cognitive status, age, and APOE-4 genetic risk on brain FDDNP positron-emission tomography imaging in persons without dementia. Arch Gen Psychiatry. 2009;66:81–87. doi: 10.1001/archgenpsychiatry.2008.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Morris JC, Roe CM, Grant EA, Head D, Storandt M, Goate AM, Fagan AM, Holtzman DM, Mintun MA. Pittsburgh Compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol. 2009;66:1469–1475. doi: 10.1001/archneurol.2009.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fagan AM, Head D, Shah AR, Marcus D, Mintun M, Morris JC, Holtzman DM. Decreased cerebrospinal fluid Abeta(42) correlates with brain atrophy in cognitively normal elderly. Ann Neurol. 2009;65:176–183. doi: 10.1002/ana.21559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Petrie EC, Cross DJ, Galasko D, Schellenberg GD, Raskind MA, Peskind ER, Minoshima S. Preclinical evidence of Alzheimer changes: convergent cerebrospinal fluid biomarker and fluorodeoxyglucose positron emission tomography findings. Arch Neurol. 2009;66:632–637. doi: 10.1001/archneurol.2009.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Storandt M, Mintun MA, Head D, Morris JC. Cognitive decline and brain volume loss as signatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Abeta deposition. Arch Neurol. 2009;66:1476–1481. doi: 10.1001/archneurol.2009.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bourgeat P, Chetelat G, Villemagne VL, Fripp J, Raniga P, Pike K, Acosta O, Szoeke C, Ourselin S, Ames D, Ellis KA, Martins RN, Masters CL, Rowe CC, Salvado O. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- 155.Oh H, Mormino EC, Madison C, Hayenga A, Smiljic A, Jagust WJ. beta-Amyloid affects frontal and posterior brain networks in normal aging. Neuroimage. 2011;54:1887–1895. doi: 10.1016/j.neuroimage.2010.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dickerson BC, Bakkour A, Salat DH, Feczko E, Pacheco J, Greve DN, Grodstein F, Wright CI, Blacker D, Rosas HD, Sperling RA, Atri A, Growdon JH, Hyman BT, Morris JC, Fischl B, Buckner RL. The cortical signature of Alzheimer's disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb Cortex. 2009;19:497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sheline YI, Raichle ME, Snyder AZ, Morris JC, Head D, Wang S, Mintun MA. Amyloid Plaques Disrupt Resting State Default Mode Network Connectivity in Cognitively Normal Elderly. Biol Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, Hedden T, Buckner RL, Becker JA, Johnson KA. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron. 2009;63:178–188. doi: 10.1016/j.neuron.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hedden T, Van Dijk KR, Becker JA, Mehta A, Sperling RA, Johnson KA, Buckner RL. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J Neurosci. 2009;29:12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mormino EC, Smiljic A, Hayenga AO, S HO, Greicius MD, Rabinovici GD, Janabi M, Baker SL, I VY, Madison CM, Miller BL, Jagust WJ. Relationships between Beta-Amyloid and Functional Connectivity in Different Components of the Default Mode Network in Aging. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Vannini P, Hedden T, Becker JA, Sullivan C, Putcha D, Rentz D, Johnson KA, Sperling RA. Age and amyloid-related alterations in default network habituation to stimulus repetition. Neurobiol Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rentz DM, Amariglio RE, Becker JA, Frey M, Olson LE, Frishe K, Carmasin J, Maye JE, Johnson KA, Sperling RA. Face-name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia. 2011;49:2776–2783. doi: 10.1016/j.neuropsychologia.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chetelat G, Villemagne VL, Pike KE, Ellis KA, Bourgeat P, Jones G, O’Keefe GJ, Salvado O, Szoeke C, Martins RN, Ames D, Masters CL, Rowe CC. Independent contribution of temporal beta-amyloid deposition to memory decline in the pre-dementiaphase of Alzheimer's disease. Brain. 2011;134:798–807. doi: 10.1093/brain/awq383. [DOI] [PubMed] [Google Scholar]
- 164.Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- 165.Rentz DM, Locascio JJ, Becker JA, Moran EK, Eng E, Buckner RL, Sperling RA, Johnson KA. Cognition, reserve, and amyloid deposition in normal aging. Ann Neurol. 2010;67:353–364. doi: 10.1002/ana.21904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liang KY, Mintun MA, Fagan AM, Goate AM, Bugg JM, Holtzman DM, Morris JC, Head D. Exercise and Alzheimer's disease biomarkers in cognitively normal older adults. Ann Neurol. 2010;68:311–318. doi: 10.1002/ana.22096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nat Rev Neurosci. 2008;9:58–65. doi: 10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 168.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M, Lyketsos CG. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161:639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 169.Rockwood K, Middleton L. Physical activity and the maintenance of cognitive function. Alzheimers Dement. 2007;3:S38–44. doi: 10.1016/j.jalz.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 170.Adlard PA, Perreau VM, Pop V, Cotman CW. Voluntary exercise decreases amyloid load in a transgenic model of Alzheimer's disease. J Neurosci. 2005;25:4217–4221. doi: 10.1523/JNEUROSCI.0496-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Leem YH, Lim HJ, Shim SB, Cho JY, Kim BS, Han PL. Repression of tau hyperphosphorylation by chronic endurance exercise in aged transgenic mouse model of tauopathies. J Neurosci Res. 2009;87:2561–2570. doi: 10.1002/jnr.22075. [DOI] [PubMed] [Google Scholar]
- 172.Wolf SA, Kronenberg G, Lehmann K, Blankenship A, Overall R, Staufenbiel M, Kempermann G. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer's disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 173.Honea RA, Thomas GP, Harsha A, Anderson HS, Donnelly JE, Brooks WM, Burns JM. Cardiorespiratory fitness and preserved medial temporal lobe volume in Alzheimer disease. Alzheimer Dis Assoc Disord. 2009;23:188–197. doi: 10.1097/WAD.0b013e31819cb8a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Roe CM, Mintun MA, Ghoshal N, Williams MM, Grant EA, Marcus DS, Morris JC. Alzheimer disease identification using amyloid imaging and reserve variables: proof of concept. Neurology. 2010;75:42–48. doi: 10.1212/WNL.0b013e3181e620f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xiong C, van Belle G, Miller JP, Morris JC. Designing clinical trials to test disease-modifying agents: application to the treatment trials of Alzheimer's disease. Clin Trials. 2011;8:15–26. doi: 10.1177/1740774510392391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mormino EC, Kluth JT, Madison CM, Rabinovici GD, Baker SL, Miller BL, Koeppe RA, Mathis CA, Weiner MW, Jagust WJ. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain. 2009;132:1310–1323. doi: 10.1093/brain/awn320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR., Jr MRI and CSF biomarkers in normal, MCI, and AD subjects: diagnostic discrimination and cognitive correlations. Neurology. 2009;73:287–293. doi: 10.1212/WNL.0b013e3181af79e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Vemuri P, Wiste HJ, Weigand SD, Shaw LM, Trojanowski JQ, Weiner MW, Knopman DS, Petersen RC, Jack CR., Jr MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology. 2009;73:294–301. doi: 10.1212/WNL.0b013e3181af79fb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Vemuri P, Wiste HJ, Weigand SD, Knopman DS, Shaw LM, Trojanowski JQ, Aisen PS, Weiner M, Petersen RC, Jack CR., Jr Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol. 2010;67:308–316. doi: 10.1002/ana.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Vemuri P, Weigand SD, Przybelski SA, Knopman DS, Smith GE, Trojanowski JQ, Shaw LM, Decarli CS, Carmichael O, Bernstein MA, Aisen PS, Weiner M, Petersen RC, Jack CR., Jr Cognitive reserve and Alzheimer's disease biomarkers are independent determinants of cognition. Brain. 2011;134:1479–1492. doi: 10.1093/brain/awr049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Jack CR, Jr, Bernstein MA, Borowski BJ, Gunter JL, Fox NC, Thompson PM, Schuff N, Krueger G, Killiany RJ, Decarli CS, Dale AM, Carmichael OW, Tosun D, Weiner MW. Update on the magnetic resonance imaging core of the Alzheimer's disease neuroimaging initiative. Alzheimers Dement. 2010;6:212–220. doi: 10.1016/j.jalz.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weiner MW, Aisen PS, Jack CR, Jr, Jagust WJ, Trojanowski JQ, Shaw L, Saykin AJ, Morris JC, Cairns N, Beckett LA, Toga A, Green R, Walter S, Soares H, Snyder P, Siemers E, Potter W, Cole PE, Schmidt M. The Alzheimer's disease neuroimaging initiative: progress report and future plans. Alzheimers Dement. 2010;6:202–211. doi: 10.1016/j.jalz.2010.03.007. e207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lo RY, Hubbard AE, Shaw LM, Trojanowski JQ, Petersen RC, Aisen PS, Weiner MW, Jagust WJ. Longitudinal Change of Biomarkers in Cognitive Decline. Arch Neurol. 2011 doi: 10.1001/archneurol.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bateman RJ, Aisen PS, De Strooper B, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimers Res Ther. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]