TREM2 and β-catenin regulate bone homeostasis by controlling the rate of osteoclastogenesis (original) (raw)
. Author manuscript; available in PMC: 2013 Aug 2.
Published in final edited form as: J Immunol. 2012 Feb 6;188(6):2612–2621. doi: 10.4049/jimmunol.1102836
Abstract
TREM2 is an immunoreceptor expressed on osteoclasts (OC) and microglia that transmit intracellular signals through the adapter DAP12. Individuals with genetic mutations inactivating TREM2 or DAP12 develop the Nasu-Hakola disease (NHD) with cystic-like lesions of the bone and brain demyelination that lead to fractures and presenile dementia. The mechanism of this disease is poorly understood. Here, we report that TREM2-deficient mice have an osteopenic phenotype reminiscent of NHD. In vitro, lack of TREM2 impairs proliferation and β-catenin activation in osteoclast precursors (OcP) in response to macrophage-colony stimulating factor (M-CSF). This defect results in accelerated differentiation of OcP into mature OC. Corroborating the importance of a balanced proliferation and differentiation of OcP for bone homeostasis, we show that conditional deletion of β-catenin in OcP also results in reduced OcP proliferation and accelerated osteoclastogenesis in vitro as well as osteopenia in vivo. These results reveal that TREM2 regulates the rate of osteoclastogenesis and provide a mechanism for the bone pathology in NHD.
Introduction
Skeletal homeostasis is sustained by a balance between bone-forming osteoblasts (OB) and bone-resorbing osteoclasts (OC) (1). While OB are of mesenchymal origin, osteoclasts (OC) originate from hematopoietic stem cells and belong to the myeloid lineage. OC differentiation and function is controlled by two major signals (2). Macrophage colony stimulating factor (M-CSF) is required for the expansion of OC precursors (OcP) and their differentiation into mature OC. RANKL is crucial for OcP differentiation into bone-resorbing OC (3).
OC differentiation and function is also controlled by cell surface receptors, including MDL-1, TREM2 and OSCAR, which transmit intracellular signals through associated transmembrane adaptors containing immunoreceptor tyrosine-based activation motifs (ITAMs) (4–11). These adaptors, including FcRγ and DAP12, recruit the protein tyrosine kinase Syk, which triggers phosphorylation events ultimately leading to Ca2+ mobilization and activation of the transcription factors NFAT, NF-κB and AP1 (12, 13). Moreover, recent evidence indicates that the receptor for M-CSF (M-CSF-R), although not physically associated with DAP12, uses this adaptor to transmit certain intracellular signals, particularly to activate Syk and β-catenin (14, 15).
FcRγ- and DAP12-associated receptors have a critical role in bone homeostasis. Mice lacking FcRγ and DAP12 have markedly reduced OC function resulting in osteopetrosis with increased bone mass and elimination of bone marrow space (16–18). In human, inactivating mutations of either TREM2 or DAP12 result in a disorder known as Nasu-Hakola disease (NHD) or polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy (PLOSL) (19, 20). NHD patients have progressive loss of white matter in the brain leading to severe presenile dementia. Moreover, they have bone lesions in the extremities that consist of areas of osteoporosis that contain poorly defined cavities filled with heterogenous lipid material (21). These lesions often lead to bone fractures following minimal trauma. Despite the established involvement of TREM2 or DAP12 mutations in NHD, the mechanism by which these molecules regulate bone remodeling in these patients remains poorly understood.
Since the TREM2/DAP12 receptor complex is expressed in OcP, it has been suggested that NHD pathological bone lesions are most likely initiated by deregulated OC generation and/or function. However, in vitro and in vivo studies of TREM2 and DAP12 function in bone homeostasis have produced unexpected results that are inconsistent with the osteporosis observed in NHD. In fact, DAP12-deficiency leads to defective osteoclast development and function in vitro and mild osteopetrosis in vivo (16, 22). Similarly, peripheral blood monocytes from both DAP12- and TREM2-deficient patients are unable to effectively generate osteoclasts with bone resorptive function in vitro (21, 23). Moreover, downregulation of TREM2 expression by RNA interference in the murine monocytic cell line RAW264.7 results in defective differentiation of this cell line into osteoclasts with bone resorbing function (24). Based on these experiments, lack of TREM2/DAP12 should result in increased bone mass.
In this study, we analyzed the bone phenotype of TREM2−/− mice and found that these animals exhibit osteoporosis and hence may provide a more accurate mouse model of the bone pathology of NHD. Mechanistically, we demonstrate that TREM2 deficiency results in a defective activation of β-catenin and proliferation of OcP, which accelerates their differentiation into functionally mature OC with bone resorbing capacity. Thus, TREM2 and β-catenin modulate bone resorption by controlling the rate of osteoclast generation.
Materials and methods
Mice and analysis of bone phenotype
Wild type, TREM2−/−, LysM-Cre and β-cateninflox/flox mice were on a C57BL/6 background, and were born and maintained under specific pathogen-free conditions in the animal care unit of Washington University School of Medicine according to guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care. Histomorphometric and microcomputed tomographic (µCT) examinations of the long bones were performed essentially as described (17, 25). Male and female mice were studied in all experiments obtaining similar results; data obtained from females are shown.
Reagents
Cell culture media were obtained from Invitrogen; fetal bovine serum was purchased from Hyclone. RANKL was kindly provided by D. Fremont (Washington University in St Louis). Mouse M-CSF was from R&D. Commercially available kits were used to measure the levels of collagen degradation products in cell culture supernatants (Nordic Bioscience Diagnostic), to measure apoptosis of the cultures (In Situ Cell Death Detection Kit, Roche) and to stain osteoclastogenic cultures for TRAP Sigma).
OcP culture and osteoclastogenesis
Bone marrow cells were cultured for 12 h in Petri dishes to remove adherent cells. Non adherent cells were transferred to new dishes and cultured in complete α-MEM (Invitrogen) containing 10% fetal bovine serum (Hyclone), and 1/10 volume of CMG 14–12 cell culture supernatant as source of M-CSF, as previously described (26). Alternatively, mouse recombinat M-CSF (R&D) was used at 100 ng/ml. At day 3, cells were considered as OcP. To generate mature OC, OcP were cultured at a density of 3000 cells/well in 96-well plates or 15,000 cells/well in 24-well plates in complete α-MEM with 10 ng/ml M-CSF and 100 ng/ml RANKL for varying times, with media being changed every 2 days.
RNA isolation and quantitative PCR
Total RNA was extracted from osteoclastogenic cultures at different timepoints using the Trizol reagent (Invitrogen). After first-strand cDNA synthesis using SuperScript III Kit (Invitrogen), real-time quantitative PCR reactions were performed for Nfatc1, Acp5, Ctsk, Calcr and Ccnd1 as previously described (14, 27). Relative quantification of target mRNA expression was calculated and normalized to the expression of cyclophilin and expressed as (mRNA of the target gene/mRNA of cyclophilin) × 106.
TRAP activity assay
A quantitative TRAP solution assay was performed by adding a colorimetric substrate, 5.5 mM _P_-nitrophenyl phosphate, in the presence of 10 mM sodium tartrate at pH 4.5. The reaction product was quantified by measuring optical absorbance at 405 nm.
Proliferation assay
M-CSF-stimulated cells were labeled with 50 µg/ml 5-bromodeoxyuridine (BrdU) for 12 h, and BrdU incorporation was measured by flow cytometry using a FITC BrdU Flow kit (BD Pharmingen). Cell growth of the 96-well plate cultures was measured by Thiazolyl Blue Tetrazolium Bromide (MTT) incorporation. In brief, MTT (Sigma) was added to the cultures at varying times at 50 µg/ml. Cells were incubated at 37°C, 5% CO2 for 4 h, media was removed and cells lysed with 200 µl of DMSO. The MTT metabolic product formazan was quantified by measuring optical absorbance at 560 nm and subtracting background at 670 nm.
Immunofluorescence and bone resorptive pits stain
Osteoclasts cultured on bovine bone slices were fixed in 4% paraformaldehyde in PBS for 20 min, permeabilized with 0.1% Triton X-100 for 30 min, and blocked with 2% FCS, 2% BSA in PBS for 1h. F-actin was stained with Alexa Fluor 488-phalloidin (Invitrogen). To stain for resorption lacunae, cells were removed from bone slices by mechanical agitation. Bone slices were incubated with peroxidase-conjugated wheat germ agglutinin (Sigma) for 1 h and stained with 3,3’-diaminobenzidine (Sigma).
Immunoprecipitation and immunoblotting
OcP were kept from M-CSF for 4 h in medium containing or not 10% fetal calf serum, harvested with PBS 3 mM EDTA, carefully counted, and replated onto tissue culture plates. Cells previously starved in medium containing serum or without it were stimulated with M-CSF or RANKL respectively (both at 100 ng/ml) for the appropriate times, lysed and immunoblotted as previously described (14). For immunoprecipitation of Syk, 5×106 cell were immunoprecipitated with anti-Syk Ab (N-19) and protein A Sepharose (GE Healthcare) and subsequently immunoblotted. Antibodies directed to the following molecules were used: PLCγ2 (3872), phospho-PLCγ2 (3874), phospho-c-Cbl (3555), phospho-shc (2434), phospho-ERK (9101), p38 (9212), phospho-p38 (9211), Akt (9272), phospho-Akt (9271), JNK (9252), phospho-JNK (9251), phospho-p65 (3033), phospho-c-Jun (2361), IκBα(9242), phospho-IκBα (5A5), Syk (2712), β-catenin (9562) and GAPDH (14C10) (Cell Signaling Technology); phospho-tyrosine (4G10, Upstate); ERK-2 (C-14), β-actin (C-11), Syk (N-19), and Lamin B (C-20) (Santa Cruz Biotechnology); phospho-Pyk2 (44–632, BioSource); Pyk2 (610548, BD Transduction Labs).
Statistics
Statistical significance was determined using GraphPad Prism, version 4.0c. Statistical differences were determined by 2-tailed Student’s t test (between 2 groups) and a 1-way ANOVA (among multiple groups). (*, P < 0.05; **, P < 0.01).
Results
TREM2−/− mice are osteopenic
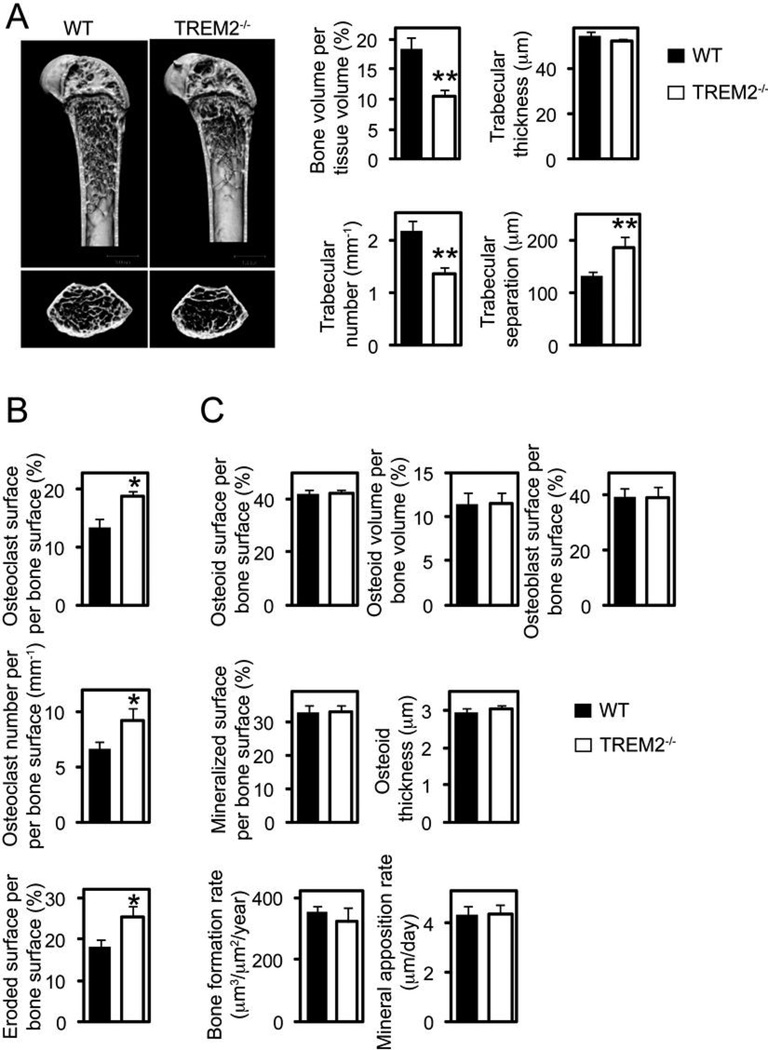
To elucidate the role of TREM2 in bone homeostasis, we analyzed the bone characteristics of TREM2−/− mice. There were no gross abnormalities in skeletal development, but microcomputed tomographic (µCT) analyses of the long bones revealed osteopenia, reduced bone volume and trabecular number, as well as increased trabecular separation in comparison to wild type mice (Fig. 1_A_). Notably, we observed by histomorphometric analysis more OC and larger OC surface and eroded surface areas in these mice (Fig. 1_B_). In contrast, OB and dynamic bone formation parameters were very similar in TREM2−/− and wild type mice (Fig. 1_C_). Thus, the osteopenic phenotype was not due to a reduction in OB numbers or activity. Collectively, these results indicate that during in vivo bone remodeling, TREM2 deficiency leads to a decrease in bone mass most likely due to enhanced OC formation and, consequently, augmented bone resorption.
Figure 1.
TREM2 deficiency results in an osteopenic phenotype. (A) Microcomputed tomography (µCT) analysis of the femurs of WT and TREM2−/− deficient mice. Upper photographs: longitudinal view; lower photographs: axial view of the metaphyseal region. The parameters are based on the µCT analysis of the metaphyseal region of mice at the age of 8 weeks. (B) osteoclastic and (C) osteoblastic parameters were obtained from bone morphometric analysis of mice at the age of 8 weeks. Graphs show mean ± SEM, N=5. *, P < 0.05; **, P < 0.01.
TREM2 deficiency accelerates OC formation in vitro
To examine the impact of TREM2 deficiency on osteoclastogenesis, we cultured TREM2−/− and WT bone marrow cells depleted of stromal cells with M-CSF for 72 h to generate OcP. OcP were then cultured with M-CSF and RANKL to induce the differentiation of OcP into OC, identified as cells that contain more than 3 nuclei and express the tartrate-resistant acid phosphatase (TRAP). We found that TREM2−/− OcP differentiated into multinuclear OC more rapidly than wild-type OcP (Fig. 2_A_ and B). Consistent with this result, TREM2−/− cultures showed more expression of OC-specific genes Nfatc1, Acp5, Ctsk and Calcr as compared to wild-type cultures 2 days after addition of RANKL (Fig. 2_C_). This difference was transient; after 4 days of culture with RANKL, there were no differences in expression of OC-specific genes. TRAP activity was also increased in TREM2−/− cells after 3 days of culture (Fig. 2_D_). OC cultured in vitro on plastic dishes are short lived and die by apoptosis. We found that TREM2−/− OC not only formed earlier but also died earlier (Fig. 2_E_). We conclude that TREM2 deficiency causes accelerated osteoclastogenesis in vitro.
Figure 2.
TREM2-deficient osteoclast precursors exhibit accelerated osteoclastogenesis. OcP generated from WT and TREM2−/− mice were cultured in vitro with 10 ng/ml M-CSF and 100 ng/ml RANKL to generate multinuclear osteoclasts. (A and B) Development of osteoclasts was monitored at different timepoints by TRAP staining. (A) Representative TRAP-stained images of the cultures. (B) The number of TRAP-positive cells containing three or more nuclei was scored (total). In addition, the nuclei in each osteoclast was enumerated as follows: 3–10; 6–10 and >10 nuclei per TRAP-positive cell. (C) Effect of TREM2 deficiency on the expression of the osteoclastic differentiation markers Nfatc1 (encoding NFATc1), Acp5 (encoding TRAP), Ctsk (encoding cathepsin K), and Calcr (encoding calcitonin receptor) measured by Q-PCR. (D) Quantification of TRAP activity in WT and TREM2−/− OcP and OC obtained by culturing OcP for 3 days with M-CSF and RANKL. (E) Apoptosis of osteoclast cultures as determined by quantification of DNA fragments by ELISA. (F, G and H) Osteoclast differentiation induced on bone slices. (F) After 4 and 7 days of differentiation cultures were fixed and stained with Phalloidin-FITC to visualize the osteoclast actin rings. (G) Bone resorption pits were revealed by lectin staining of the bone slices (brown reaction product). (H) Supernatants of the cultures were collected at day 4 and 6 of culture and bone resorption was assessed by measuring the degradation products of the C-terminal telopeptides of Type I collagen by ELISA. *, P < 0.05; **, P < 0.01. Results are representative of at least 3 separate experiments.
We also examined the ability of TREM2−/− OcP to differentiate into OC when cultured on a mineralized substrate. OC generated by this method are more similar to OC in vivo, as their actin cytoskeleton forms in typical actin rings. Similar to cultures generated on tissue culture plastic, many more OC were formed on bone slices from TREM2−/− OcP compared to wild-type cells as early as 4 days of culture, although no differences in numbers were noted after 7 days of culture (Fig. 2_F_). Accelerated osteoclastogenesis was associated with increased numbers of resorption pits after 4 days, but not after 7 days of culture (Fig. 2_G_). Bone degradation, measured as the accumulation of type I collagen fragments in the culture media, was also significantly higher in the TREM2−/− cultures at early timepoints (Fig. 2_H_). Therefore, TREM2 deficiency also causes accelerated differentiation of OC on a mineralized substrate. Together, these results indicate that TREM2 negatively regulates OC formation at an early stage, but has no impact on the function of mature OC.
TREM2-deficiency does not augment M-CSF and RANK signaling
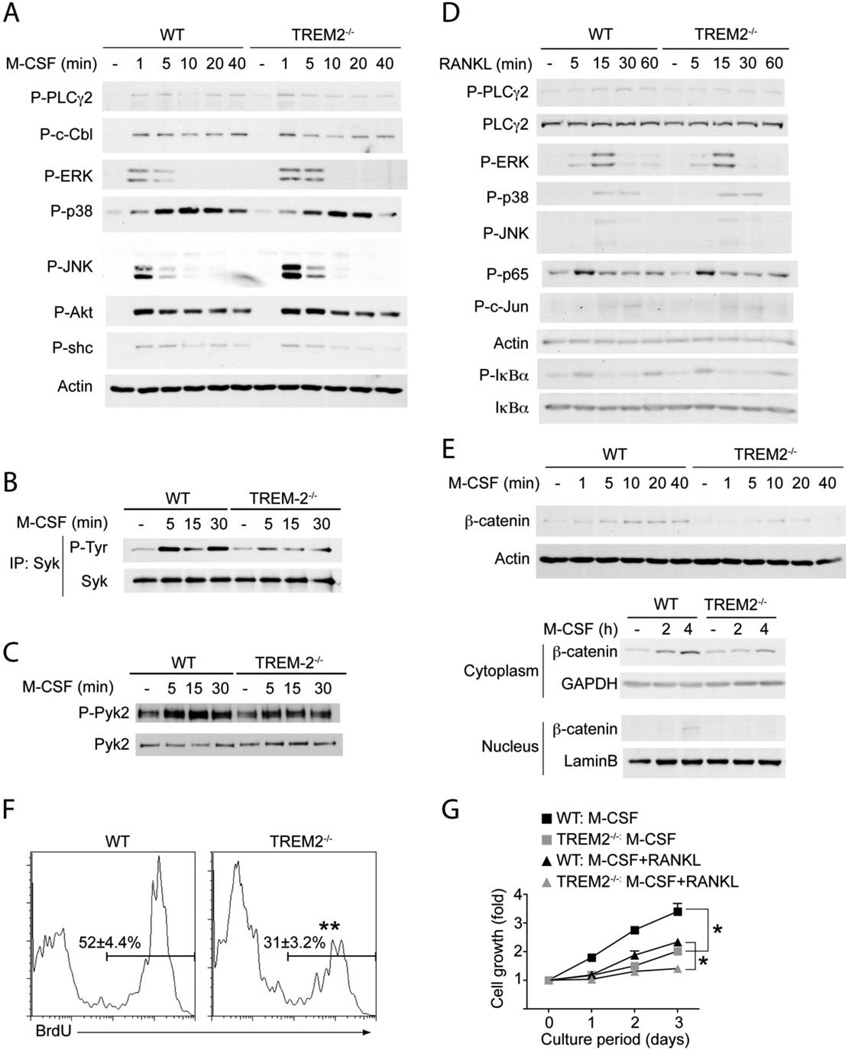
It was reported that engagement of DAP12-associated receptors with low avidity ligands induce a partial phosphorylation of DAP12, leading to DAP12 recruitment of inhibitory mediators, such as the protein tyrosine phosphatases SHP-1 (28) or the lipid phosphatase SHIP (29). The abnormal recruitment of phosphatases in place of the canonical recruitment of the protein tyrosine kinase Syk may result in inhibition of heterologous receptor signaling pathways. Thus, we tested the hypothesis that TREM2 delivers inhibitory signals during bone homeostasis and that accelerated osteoclastogenesis of TREM2−/− OcP depends on the release of M-CSF and/or RANKL signaling from TREM2-mediated inhibition. TREM2−/− and WT OcP were cultured in M-CSF for 72–96 h, deprived of M-CSF for few hours and then left untreated or stimulated with M-CSF for increasing periods of time. Cell lysates were analyzed for phosphorylation of several downstream effectors, including the MAPK ERK, JNK and p38, the adaptors c-Cbl and shc, as well as Syk, PLCγ2 and Akt. M-CSF-induced phosphorylation of most mediators was very similar in TREM2−/− and WT OcP, with the exception of a slight but consistent increase of ERK phosphorylation in TREM2−/− OcP, reminiscent of what previously observed in DAP12-deficient macrophages (14) (Fig. 3_A_). Notably, phosphorylation of Syk, a known target of DAP12 that has an essential role in osteoclastogenesis (18), was reduced in TREM2−/− OcP (Fig. 3_B_). Additionally, phosphorylation of Pyk2, a downstream target of Syk, was slightly but reproducibly reduced in TREM2−/− OcP compared with WT OcP (Fig. 3C). These results suggest that TREM-2 does not recruit tyrosine phosphatases. Lack of TREM2 did not abolish basal Syk phosphorylation, most likely because TREM2-deficient OC expressed other DAP12-associated receptors, such as Sirpβ1 and Clec5A, which can transduce ITAM signals (Fig. S1).
Figure 3.
Impaired M-CSF-induced β-catenin expression and cell proliferation of TREM2-deficient osteoclast precursors. (A–E) OcP generated from WT and TREM2−/− mice were starved from M-CSF for 4 h and then exposed to 50 ng/ml M-CSF (A, B, C and E) or were starved from M-CSF and serum for 4 h and then exposed to 100 ng/ml RANKL (D). After the indicated times total cell lysates were prepared and subjected to immunoprecipitation and/or immunoblotting analysis using antibodies to the indicated proteins. Actin served as loading control for total lysates. (A) M-CSF-induced phosphorylation of various signaling mediators. (B) TREM2-dependent activation of Syk by M-CSF. After the indicated times of M-CSF stimulation total cell lysates were prepared and immunoprecipitated (IP) with anti-Syk Ab. Immunoblots were performed with anti-phosphotyrosine Ab to detect activated Syk. To control for protein loading, the membranes were reprobed for total Syk. (C) TREM2-dependent activation of Pyk2 by M-CSF. Immunoblots of total cell lysates were performed with anti-phospho-Pyk2 and anti-Pyk2. (D) RANKL-induced phosphorylation of various signaling mediators. (E) M-CSF-induced β-catenin expression and its nuclear translocation is dependent of TREM2. OcP were cultured in the absence of M-CSF for 4 h and then restimulated with M-CSF (100 ng/ml) and the expression of β-catenin in total lysates, cytoplasmic and nuclear cell fractions was analyzed by inmunoblotting. Actin, GAPDH and lamin-B served as loading controls for total cell lysates, cytoplasmic and nuclear cell fractions respectively. (F) Defect of M-CSF-induced proliferation in TREM2-deficient OcP. Proliferation of WT and TREM2−/− OcP was measured 12 h after incubation with BrdU in the presence of 100 ng/ml M-CSF. (G) Time course of changes in cell growth in OcP cultured in the presence of osteoclastogenic cytokines. OcP were cultured with 100 ng/ml M-CSF with or without 100 ng/ml RANKL. After culturing for the indicating periods, cell growth was measured by the MTT assay and expressed as the increase in OD560nm -background OD670nm relative to day 0. *, P < 0.05; **, P < 0.01. Results are representative of at least 3 separate experiments.
We also examined activation of signaling mediators in TREM2−/− and WT OcP cultured in M-CSF for 72–96 hrs and stimulated with RANKL. RANK-induced activation of PLCγ2, MAPKs, p65, c-jun and IκBα were comparable in TREM2−/− and WT cells (Fig. 3_D_). We conclude that TREM2 does not elicit inhibitory signals through DAP12 and that accelerated osteoclastogenesis in TREM2−/− mice is not due to an increase of M-CSF or RANK signaling.
TREM2-deficiency impairs M-CSF-induced activation β-catenin and OcP proliferation
We previously reported that M-CSF signaling promotes expression and nuclear translocation of β-catenin and that DAP12 enhances M-CSF-induced activation of β-catenin (14). Thus, we asked whether TREM2-deficiency affects this pathway. After stimulation with M-CSF, TREM2−/− and WT OcP were examined for expression of β-catenin in the total cell lysates as well as in isolated cytosolic and nuclear extracts. While basal β-catenin levels were similar in TREM2−/− and WT OcP, M-CSF-induced expression of β-catenin in total cell lysates, cytosolic accumulation and nuclear translocation of β-catenin were all reduced in the absence of TREM2 (Fig 3_E_). In addition, TREM2−/− OC expressed less cyclin D1 mRNA, a known β-catenin target gene, than WT OC (Fig. S1).
Because β-catenin plays an essential role in proliferation, we measured proliferation and cell growth in TREM2−/− and WT OcP cultures by BrdU incorporation and MTT assays respectively. TREM2−/− OcP showed a significant reduction of proliferation (Fig. 3_F_). As previously reported, M-CSF alone promoted cell proliferation of WT OcP, which was reduced by addition of RANKL (30, 31) (Fig. 3_G_). Notably, TREM2−/− OcP cultured with M-CSF alone or both M-CSF and RANKL proliferated significantly less than wild-type OcP (Fig. 3_G_). We conclude that TREM2 is essential for enhancing M-CSF-induced proliferation of OcP, possibly by facilitating the activation of β-catenin. OcP, like other precursors cells, undergo cell cycle arrest prior to differentiation into mature OC (30). Given this, it is conceivable that TREM2 deficiency may induce an earlier cell cycle arrest, which favors OcP to differentiate more rapidly into mature OC.
Mice lacking β-catenin in the OcP compartment are osteopenic
The osteopenic phenotype observed in TREM2−/− mice associated with the reduced activation of β-catenin suggested the intriguing possibility that β-catenin could be essential for regulating osteoclastogenesis. To investigate the effect of β-catenin in OC differentiation in a cell-autonomous fashion, we generated mice with a selective deletion of β-catenin in OcP. Mice harboring a floxed β-catenin allele (βcatflox/+) were crossed with mice expressing the Cre recombinase under the control of regulatory elements of the LysM gene (LysMCre/Cre). LysMCre/+βcatflox/+ were then crossed with LysMCre/Cre, and the LysMCre/Creβcatflox/+ obtained were intercrossed to generate LysMCre/Creβcatflox/flox β-catenin deficient mice (βcatΔ̃Δ). LysMCre/Creβcat+/+ (βcat+/+) littermates served as controls.
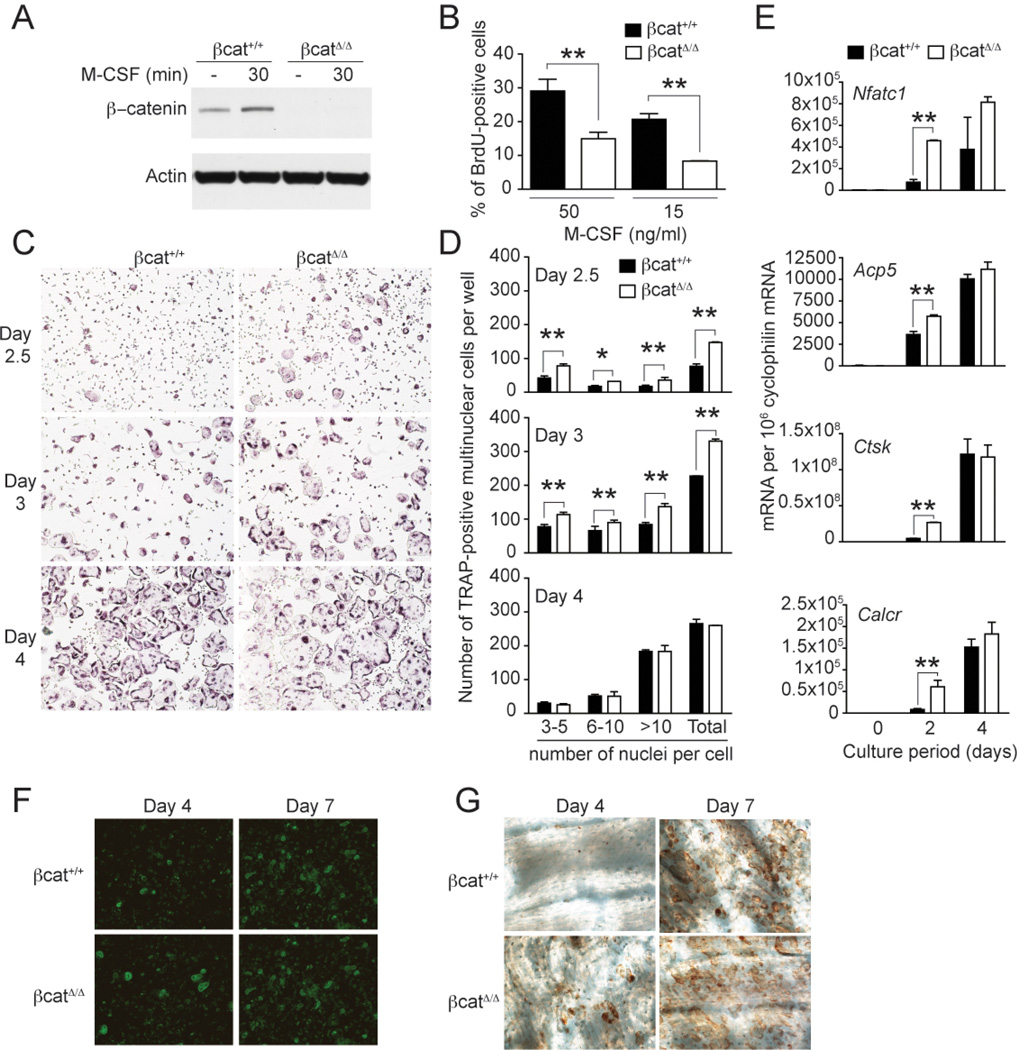
Immunoblot analysis of OcP derived in vitro from βcatΔ̃Δ bone marrow cells confirmed complete deletion of β-catenin expression (Fig. 4_A_). CD11b, F4/80, MHCII and CD115 were equally expressed in βcatΔ̃Δ and βcat+/+ OcP, suggesting that β -catenin deficiency does not affect the differentiation of bone marrow precursor cells into OcP (data not shown). However, βcatΔ̃Δ OcP proliferated less than βcat+/+ OcP in response to M-CSF (Fig. 4_B_). Moreover, βcatΔ̃Δ OcP formed mature OC more rapidly than βcat+/+ OcP during culture with M-CSF and RANKL (Fig. 4_C_ and D). Mature, terminally differentiated βcatΔ̃Δ OC had no obvious defects in size or morphology. Consistent with accelerated ostoclastogenesis, acquisition of the osteoclast-specific genes Nfatc1, Acp5, Ctsk and Calcr was more rapid in βcatΔ̃Δ OC as compared to βcat+/+ OC at early timepoints during culture (Fig. 4_E_). When OcP were differentiated on bone slices, βcatΔ̃Δ OcP formed more OC compared to βcat+/+ cells after 4 days of culture, although no differences in numbers were found after 7 days (Fig. 4_F_). Accelerated osteoclastogenesis of βcatΔ̃Δ OC was associated with increased numbers of resorption pits after 4 days, but not after 7 days of culture (Fig. 4_G_). No major differences in the activation of classical downstream mediators were observed following analysis M-CSF- and RANKL-induced signals in βcatΔ̃Δ and βcat+/+ OcP (Fig. S2). Together, these results indicate that β-catenin has a cell-autonomous function at the early stages of OC generation, sustaining M-CSF-induced proliferation of OcP, but preventing their differentiation into mature OC. In contrast, β-catenin has no impact on the function of mature OC once they are generated.
Figure 4.
β-catenin-deficient osteoclast precursors proliferate less to M-CSF and exhibit accelerated osteoclastogenesis. (A) Immunoblot analysis of β-catenin and actin (loading control) in OcP total protein lysates from control LysM-Cre+/+ β-catenin+/+ (β-cat+/+) and LysM-Cre+/+ β-cateninfl/fl (β-catΔ̃Δ mice. (B) Defect of M-CSF-induced proliferation in β-catenin-deficient OcP. Proliferation of β-cat+/+ and β-catΔ̃Δ OcP was measured 12 h after incubation with BrdU in the presence of 100 ng/ml M-CSF. (C and D) OcP from β-cat+/+ and β-catΔ̃Δ mice were cultured with 10 ng/ml M-CSF and 100 ng/ml RANKL to generate multinuclear osteoclasts. Development of osteoclasts was monitored at different timepoints by TRAP staining. (C) Representative images of TRAP-stained cultures. (D) The number of TRAP-positive cells containing three or more nuclei was scored (total). In addition, the nuclei in each osteoclast was enumerated as follows: 3–10; 6–10 and >10 nuclei per TRAP-positive cell. (E) Effect of β-catenin deficiency on the expression of the osteoclastic differentiation markers Nfatc1 (encoding NFATc1), Acp5 (encoding TRAP), Ctsk (encoding cathepsin K), and Calcr (encoding calcitonin receptor) by Q-PCR. (F and G) Osteoclast differentiation induced on bone slices. (F) After 4 and 7 days of differentiation cultures were fixed and stained with Phalloidin-FITC to visualize the osteoclast actin rings. (G) Bone resorption pits were revealed by lectin staining of the bone slices (brown reaction product). *, P < 0.05; **, P < 0.01. Results are representative of at least 3 separate experiments.
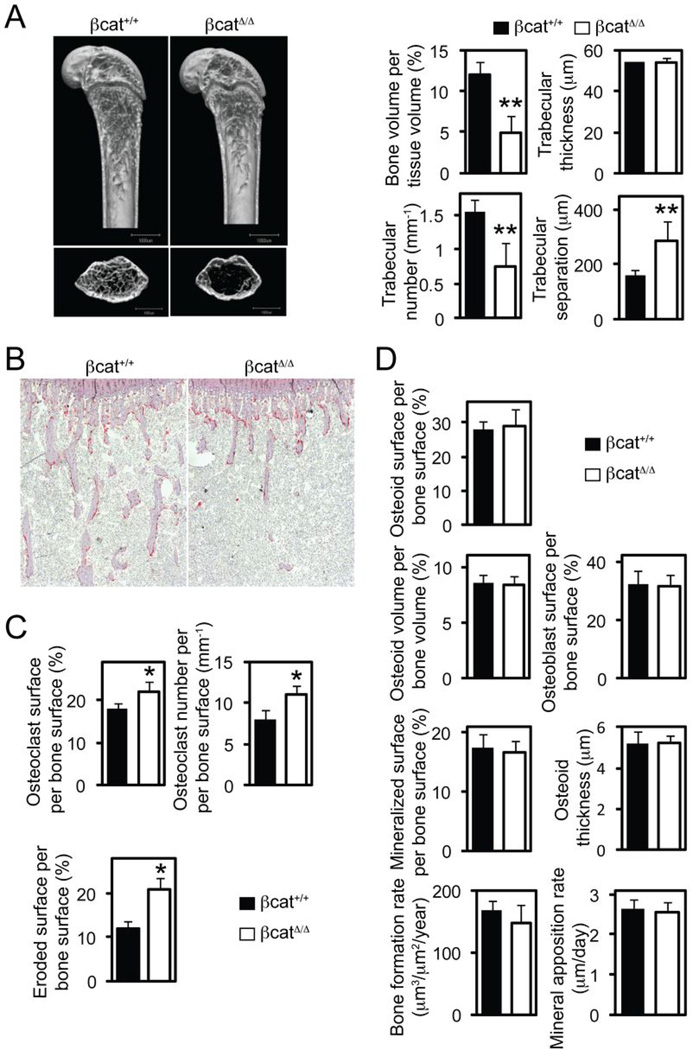
Consistent with the inhibitory role of β-catenin in the in vitro OC differentiation, µCT and histomorphometry analysis revealed a decrease in trabecular bone mass in βcatΔ̃Δ mice compared to control mice (Fig. 5_A_). TRAP staining of long bone sections demonstrated significant increase in OC numbers, OC surface and eroded surface areas in βcatΔ̃Δ mice (Fig. 5_B_ and C). No differences in OB and dynamic bone formation parameters were noted following bone-morphometric analysis (Fig. 5_D_), indicating that β-catenin deletion in the βcatΔ̃Δ mice does not affect OB numbers or function. We conclude that β-catenin in the OC lineage plays an essential role in controlling early OC differentiation and bone homeostasis.
Figure 5.
Mice deficient for β-catenin in osteoclast precursors are osteopenic. (A) Microcomputed tomography (µCT) analysis of the femurs of LysM-Cre+/+ β-catenin+/+ (β-cat+/+) and LysM-Cre+/+ β-cateninfl/fl (β-catΔ̃Δ mice. Upper photographs: longitudinal view; lower photographs: axial view of the metaphyseal region. The parameters are based on the µCT analysis of the metaphyseal region of mice at the age of 6 weeks. (B–D) Histological analysis of the tibiae. (B) Photographs represent TRAP and hematoxilin staining. (C) Osteoclastic and (D) osteoblastic parameters were obtained from bone morphometric analysis of mice at the age of 6 weeks. Graphs show mean ± SEM, N=5. *, P < 0.05; **, P < 0.01.
Simultaneous heterozygosity for TREM2- and β-catenin-null alleles results in osteoporosis
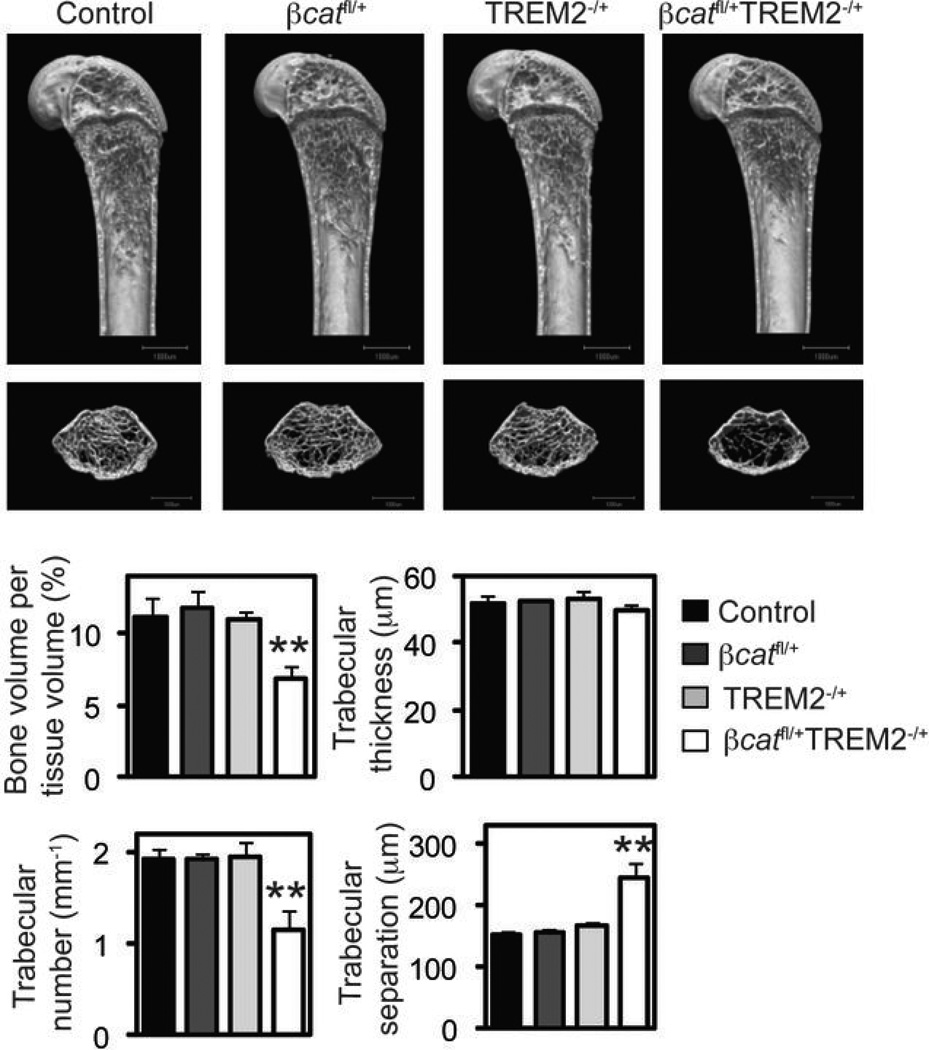
TREM2 and β-catenin deficiency cause similar bone phenotypes, and M-CSF induction of β-catenin is reduced in TREM2−/− OcP, suggesting a link between TREM2 and β-catenin. To corroborate this possibility, we generated mice double-heterozygous for TREM2 and β-catenin (βcatfl/+TREM2−/+) in the LysMCre/Cre background and analyzed their bone phenotypes. µCT analysis of the long bones showed that these mice had a significant reduction of bone volume and trabecular number, as well as a marked increase in trabecular separation compared to age- and sex-matched single heterozygous littermates, which, in turn, were not different from control WT littermates (Fig. 6). Importantly, the osteopenic phenotype of LysMCre/Creβcatfl/+TREM2−/+ mice phenocopies the TREM2−/− and the βcatΔ̃Δ mutant phenotype. These results provide a genetic basis in support of a coordinate action of TREM2 with β-catenin to modulate bone homeostasis.
Figure 6.
TREM-2 and β -catenin function together to maintain osteoclast numbers and bone homeostasis. We generated mice with the genotype LysM-Cre+/+ with the allelic combinations β-catenin+/+ Trem2+/+ (Control), β-cateninfl/+ Trem2+/+ (βcatfl/+), β-catenin+/+ Trem2−/+ (Trem2−/+), and β-cateninfl/+ Trem2−/+(βcatfl/+Trem2−/+). Femurs were analyzed by µCT. Upper photographs: longitudinal view; lower photographs: axial view of the metaphyseal region. The parameters are based on the µCT analysis of the metaphyseal region of mice at the age of 8 weeks. Graphs show mean ± SEM, N=5. **, P < 0.01.
Discussion
Our study unveils a TREM2-β-catenin pathway that regulates bone mass by regulating the rate of OC generation. Mechanistically, TREM2 and β-catenin augment the M-CSF-induced proliferation of OcP, retarding their differentiation into mature OC. Ablation of either TREM2 or β-catenin inhibits the proliferation of OcP, accelerating their differentiation into bone resorbing OC, which ultimately cause osteoporosis. The possibility that TREM2 and β-catenin act along the same pathway is supported not only by the similar osteoporotic phenotypes of TREM2−/− and βcatΔ̃Δ mice, but also by genetic evidence that simultaneous heterozygosity for TREM2 and β -catenin null alleles results in osteoporosis whereas no phenotype is observed in mice heterozygous for either of these alleles.
TREM2 may enhance M-CSF-induced activation of β-catenin by facilitating the recruitment of DAP12 to the M-CSF-R. In turn, DAP12 may activate Syk and Pyk2, which promote phosphorylation and nuclear translocation of β-catenin (14). Accordingly, we found that TREM-2 deficiency impaired the M-CSF-induced phosphorylation of Syk and, in part, Pyk2. Conversely, basal phosphorylation of Syk was preserved. M-CSF-induced activation of Syk was proposed to mediate osteoclast function (14, 15). However, the reduction of this signaling pathway in TREM2−/− mice resulted in a selective defect in OcP proliferation, whereas OC differentiation was accelerated and OC function was preserved. Therefore, we speculate that differentiation and particularly cytoskeleton rearrangement of OC may be independent of M-CSF-induced Syk activation, rather depending on the basal activation of Syk by signals emanating from integrins or other ITAM-signaling receptors.
The bone phenotype observed in TREM2−/− and βcatΔ̃Δ mice more closely resemble the bone phenotype of NHD. NHD patients have osteoporosis whether the disease is caused by inactivating mutations of TREM2 or the associated adapter DAP12. However, in mouse, only TREM2−/− mice have an osteoporotic phenotype, whereas DAP12−/− mice exhibit a mild osteopetrotic phenotype. The disparate impacts of DAP12 deficiency in humans and mice most likely reflect a difference in the spectrum of receptors involved in osteoclastogenesis and their capacity to signal through DAP12 or other ITAM-containing subunits like FcRγ. In humans, the TREM2/DAP12 complex may selectively enhance OcP proliferation, whereas OcP differentiation into mature OC may be mediated by FcRγ-associated receptors. Thus, both TREM2 and DAP12 deficiencies would result in accelerated differentiation of OC and osteoporosis in vivo. In mouse, DAP12 may control OcP proliferation through TREM2 as well as differentiation of OC through other receptors. Thus, while deficiency of TREM2 selectively affects OcP proliferation, deficiency of DAP12 may affect both OcP proliferation and mature OC functions, resulting in a mild osteopetrosis, which becomes more dramatic in mice lacking both DAP12 and FcRγ (17).
Our demonstration that TREM2-deficiency accelerates osteoclastogenesis in cultures of bone marrow cells in vitro is at odds with previous studies showing that human blood monocytes lacking TREM2 have a reduced capacity to generate functional OC in vitro (21, 23). Similarly, RNAi knockdown of TREM2 in the murine macrophage cell line RAW264.7 inhibited in vitro osteoclastogenesis (24). It is possible that human monocytes and mouse RAW264.7 cells, although capable of generating OC in vitro, differ from the physiological OcP in their spectrum of DAP12 and FcRγ-associated receptors, such that the lack or blockade of a functional TREM2/DAP12 complex has wider impact on human monocytes and RAW264.7 cells-derived OC than in OcP-derived OC. Supporting this hypothesis, several reports have shown that modulation of TREM2 function in human monocytes has a major impact on DAP12-ITAM signaling (32–34). Alternatively, cultures of purified human monocytes and RAW264.7 cells may lack ligands capable of engaging DAP12 and/or FcRγ-associated receptors. Regardless of the cause, TREM2−/− bone marrow cultures and TREM2−/− mice seem to provide the best models for in vitro and in vivo studies of NHD bone pathology.
Although the role of β-catenin in osteoblasts development and function is well established (35–38), little is known about its function in osteoclasts. A recent study proposed that β-catenin regulates osteoclastogenesis in a dosage-dependent fashion such that β-catenin can have either an activating or inhibitory function (39). Our study suggests that activation of TREM2-β-catenin pathway in OcP may reduce osteoclastogenesis. Thus, from a therapeutic perspective, activation of the β-catenin pathway in bone cells could be a valuable strategy in the treatment of osteoporosis, as it would uniquely combine simultaneous stimulation of osteoblasts with inhibition of osteoclastogenesis. However, systemic activation of β-catenin may have important drawbacks. It has been shown that continuous activation of β-catenin in the hematopoietic system causes excessive proliferation and exhaustion of the hematopoietic progenitor pool, leading to a failure in the generation of myeloid and lymphoid lineages (40, 41). Moreover β-catenin is key to balancing tolerance and immunity through the control of dendritic cell function (42, 43). Because of TREM2 restricted expression, activating β-catenin downstream of TREM2 may provide a good strategy to selectively activate β-catenin in the osteoclast lineage for increasing bone mass in osteoporosis.
Supplementary Material
Supp Figs 1 and 2
Acknowledgements
We would like to thank Drs Toshihide Mizoguchi, Naoyuki Takahashi (Matsumoto Dental University) and Fanxin Long (Washington University in St. Louis) for helpful discussions; Drs Wei Zou and Viviana Cremasco (Washington University in St. Louis) for technical advice on OC generation and helpful discussions. K. O. is supported by the NRSA training grant 5T32AI7163-32
Abbreviations used in this article
OC
osteoclasts
OcP
osteoclast precursors
OB
osteoblasts
NHD
Nasu-Hakola disease
PLOSL
polycystic lipomembranous osteodysplasia with sclerosing leukoencephalopathy
µCT
microcomputed tomography
TRAP
tartrate-resistant acid phosphatase
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Raggatt LJ, Partridge NC. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010;285:25103–25108. doi: 10.1074/jbc.R109.041087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teitelbaum SL. Osteoclasts: what do they do and how do they do it? Am. J. Pathol. 2007;170:427–435. doi: 10.2353/ajpath.2007.060834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leibbrandt A, Penninger JM. RANKL/RANK as key factors for osteoclast development and bone loss in arthropathies. Adv. Exp. Med. Biol. 2009;649:100–113. doi: 10.1007/978-1-4419-0298-6_7. [DOI] [PubMed] [Google Scholar]
- 4.Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, Cella M, Kim T, Rho J, Negishi-Koga T, Delaisse JM, Takayanagi H, Lorenzo J, Colonna M, Farndale RW, Choi Y, Trowsdale J. OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice. J. Clin. Invest. 2011;121:3505–3516. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamerman JA, Ni M, Killebrew JR, Chu CL, Lowell CA. The expanding roles of ITAM adapters FcRgamma and DAP12 in myeloid cells. Immunol. Rev. 2009;232:42–58. doi: 10.1111/j.1600-065X.2009.00841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inui M, Kikuchi Y, Aoki N, Endo S, Maeda T, Sugahara-Tobinai A, Fujimura S, Nakamura A, Kumanogoh A, Colonna M, Takai T. Signal adaptor DAP10 associates with MDL-1 and triggers osteoclastogenesis in cooperation with DAP12. Proc. Natl. Acad. Sci. USA. 2009;106:4816–4821. doi: 10.1073/pnas.0900463106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivashkiv LB. Cross-regulation of signaling by ITAM-associated receptors. Nat. Immunol. 2009;10:340–347. doi: 10.1038/ni.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lanier LL. DAP10- and DAP12-associated receptors in innate immunity. Immunol. Rev. 2009;227:150–160. doi: 10.1111/j.1600-065X.2008.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee SH, Kim TS, Choi Y, Lorenzo J. Osteoimmunology: cytokines and the skeletal system. BMB Rep. 2008;41:495–510. doi: 10.5483/bmbrep.2008.41.7.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat. Rev. Rheum. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y, Humphrey MB, Nakamura MC. Osteoclasts - the innate immune cells of the bone. Autoimmunity. 2008;41:183–194. doi: 10.1080/08916930701693180. [DOI] [PubMed] [Google Scholar]
- 12.McVicar DW, Trinchieri G. CSF-1R, DAP12 and beta-catenin: a menage a trois. Nat. Immunol. 2009;10:681–683. doi: 10.1038/ni0709-681. [DOI] [PubMed] [Google Scholar]
- 13.McVicar DW, Burshtyn DN. Intracellular signaling by the killer immunoglobulin-like receptors and Ly49. Science's STKE. 2001;2001:re1. doi: 10.1126/stke.2001.75.re1. [DOI] [PubMed] [Google Scholar]
- 14.Otero K, Turnbull IR, Poliani PL, Vermi W, Cerutti E, Aoshi T, Tassi I, Takai T, Stanley SL, Miller M, Shaw AS, Colonna M. Macrophage colony-stimulating factor induces the proliferation and survival of macrophages via a pathway involving DAP12 and beta-catenin. Nat. Immunol. 2009;10:734–743. doi: 10.1038/ni.1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou W, Reeve JL, Liu Y, Teitelbaum SL, Ross FP. DAP12 couples c-Fms activation to the osteoclast cytoskeleton by recruitment of Syk. Mol. Cell. 2008;31:422–431. doi: 10.1016/j.molcel.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaifu T, Nakahara J, Inui M, Mishima K, Momiyama T, Kaji M, Sugahara A, Koito H, Ujike-Asai A, Nakamura A, Kanazawa K, Tan-Takeuchi K, Iwasaki K, Yokoyama WM, Kudo A, Fujiwara M, Asou H, Takai T. Osteopetrosis and thalamic hypomyelinosis with synaptic degeneration in DAP12-deficient mice. J. Clin. Invest. 2003;111:323–332. doi: 10.1172/JCI16923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 18.Mocsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc. Natl. Acad. Sci. USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paloneva J, Kestila M, Wu J, Salminen A, Bohling T, Ruotsalainen V, Hakola P, Bakker AB, Phillips JH, Pekkarinen P, Lanier LL, Timonen T, Peltonen L. Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat. Genet. 2000;25:357–361. doi: 10.1038/77153. [DOI] [PubMed] [Google Scholar]
- 20.Paloneva J, Manninen T, Christman G, Hovanes K, Mandelin J, Adolfsson R, Bianchin M, Bird T, Miranda R, Salmaggi A, Tranebjaerg L, Konttinen Y, Peltonen L. Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 2002;71:656–662. doi: 10.1086/342259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paloneva J, Mandelin J, Kiialainen A, Bohling T, Prudlo J, Hakola P, Haltia M, Konttinen YT, Peltonen L. DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J. Exp. Med. 2003;198:669–675. doi: 10.1084/jem.20030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Humphrey MB, Ogasawara K, Yao W, Spusta SC, Daws MR, Lane NE, Lanier LL, Nakamura MC. The signaling adapter protein DAP12 regulates multinucleation during osteoclast development. J. Bone Miner. Res. 2004;19:224–234. doi: 10.1359/JBMR.0301234. [DOI] [PubMed] [Google Scholar]
- 23.Cella M, Buonsanti C, Strader C, Kondo T, Salmaggi A, Colonna M. Impaired differentiation of osteoclasts in TREM-2-deficient individuals. J. Exp. Med. 2003;198:645–651. doi: 10.1084/jem.20022220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Humphrey MB, Daws MR, Spusta SC, Niemi EC, Torchia JA, Lanier LL, Seaman WE, Nakamura MC. TREM2, a DAP12-associated receptor, regulates osteoclast differentiation and function. J. Bone Miner. Res. 2006;21:237–245. doi: 10.1359/JBMR.051016. [DOI] [PubMed] [Google Scholar]
- 25.Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS, Kitamura D, Kurosaki T, Ellmeier W, Takayanagi H. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 26.Ito Y, Teitelbaum SL, Zou W, Zheng Y, Johnson JF, Chappel J, Ross FP, Zhao H. Cdc42 regulates bone modeling and remodeling in mice by modulating RANKL/M-CSF signaling and osteoclast polarization. J. Clin. Invest. 2010;120:1981–1993. doi: 10.1172/JCI39650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J. Clin. Invest. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakano-Yokomizo T, Tahara-Hanaoka S, Nakahashi-Oda C, Nabekura T, Tchao NK, Kadosaki M, Totsuka N, Kurita N, Nakamagoe K, Tamaoka A, Takai T, Yasui T, Kikutani H, Honda S, Shibuya K, Lanier LL, Shibuya A. The immunoreceptor adapter protein DAP12 suppresses B lymphocyte-driven adaptive immune responses. J. Exp. Med. 2011;208:1661–1671. doi: 10.1084/jem.20101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peng Q, Malhotra S, Torchia JA, Kerr WG, Coggeshall KM, Humphrey MB. TREM2- and DAP12-dependent activation of PI3K requires DAP10 and is inhibited by SHIP1. Science Signaling. 2010;3:ra38. doi: 10.1126/scisignal.2000500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mizoguchi T, Muto A, Udagawa N, Arai A, Yamashita T, Hosoya A, Ninomiya T, Nakamura H, Yamamoto Y, Kinugawa S, Nakamura M, Nakamichi Y, Kobayashi Y, Nagasawa S, Oda K, Tanaka H, Tagaya M, Penninger JM, Ito M, Takahashi N. Identification of cell cycle-arrested quiescent osteoclast precursors in vivo. J. Cell Biol. 2009;184:541–554. doi: 10.1083/jcb.200806139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sankar U, Patel K, Rosol TJ, Ostrowski MC. RANKL coordinates cell cycle withdrawal and differentiation in osteoclasts through the cyclin-dependent kinase inhibitors p27KIP1 and p21CIP1. J. Bone Miner. Res. 2004;19:1339–1348. doi: 10.1359/JBMR.040321. [DOI] [PubMed] [Google Scholar]
- 32.Park-Min KH, Ji JD, Antoniv T, Reid AC, Silver RB, Humphrey MB, Nakamura M, Ivashkiv LB. IL-10 suppresses calcium-mediated costimulation of receptor activator NF-kappa B signaling during human osteoclast differentiation by inhibiting TREM-2 expression. J. Immunol. 2009;183:2444–2455. doi: 10.4049/jimmunol.0804165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalliolias GD, Zhao B, Triantafyllopoulou A, Park-Min KH, Ivashkiv LB. Interleukin-27 inhibits human osteoclastogenesis by abrogating RANKL-mediated induction of nuclear factor of activated T cells c1 and suppressing proximal RANK signaling. Arthritis Rheum. 2010;62:402–413. doi: 10.1002/art.27200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang JH, Chang EJ, Kim HH, Kim SK. Enhanced inhibitory effects of a novel CpG motif on osteoclast differentiation via TREM-2 down-regulation. Biochem. Biophys. Res. Commun. 2009;389:28–33. doi: 10.1016/j.bbrc.2009.08.080. [DOI] [PubMed] [Google Scholar]
- 35.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 36.Takahashi N, Maeda K, Ishihara A, Uehara S, Kobayashi Y. Regulatory mechanism of osteoclastogenesis by RANKL and Wnt signals. Front. Biosci. 2011;16:21–30. doi: 10.2741/3673. [DOI] [PubMed] [Google Scholar]
- 37.Hu H, Hilton MJ, Tu X, Yu K, Ornitz DM, Long F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development. 2005;132:49–60. doi: 10.1242/dev.01564. [DOI] [PubMed] [Google Scholar]
- 38.Glass DA, 2nd, Bialek P, Ahn JD, Starbuck M, Patel MS, Clevers H, Taketo MM, Long F, McMahon AP, Lang RA, Karsenty G. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 39.Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan Y. Biphasic and dosage-dependent regulation of osteoclastogenesis by beta-catenin. Mol. Cell. Biol. 2011;31:4706–4719. doi: 10.1128/MCB.05980-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kirstetter P, Anderson K, Porse BT, Jacobsen SE, Nerlov C. Activation of the canonical Wnt pathway leads to loss of hematopoietic stem cell repopulation and multilineage differentiation block. Nat. Immunol. 2006;7:1048–1056. doi: 10.1038/ni1381. [DOI] [PubMed] [Google Scholar]
- 41.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat. Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 42.Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, Whitney JA, Connolly J, Banchereau J, Mellman I. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007;27:610–624. doi: 10.1016/j.immuni.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Manicassamy S, Reizis B, Ravindran R, Nakaya H, Salazar-Gonzalez RM, Wang YC, Pulendran B. Activation of beta-catenin in dendritic cells regulates immunity versus tolerance in the intestine. Science. 2010;329:849–853. doi: 10.1126/science.1188510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supp Figs 1 and 2