Rhinovirus-Induced IL-1β Release from Bronchial Epithelial Cells Is Independent of Functional P2X7 (original) (raw)
Abstract
Airway epithelial cell defenses to viral infections are often compromised in disease or injury. Danger molecules, including ATP, are released during infection and contribute to nucleotide receptor–dependent inflammatory responses, largely through P2X7. Although respiratory epithelium has been shown to express a variety of nucleotide receptors, the functional contribution of P2X7 to the epithelial cell inflammatory response is unclear. We used human donor bronchial epithelial cells (BECs) and primary brushed epithelium to explore responses upon nucleotide and Toll-like receptor stimulation. P2X7 messenger RNA and protein were observed in unprimed BECs, whereas inflammatory cytokine stimulation increased both messenger RNA and protein. Functional pore activity characteristic of P2X7 was observed in BECs, and IL-1β was rapidly released by BECs after Toll-like receptor 3 agonist, polyinosine-polycytidylic acid, priming followed by ATP administration, although no change was observed in IL-18 release. BECs produced more IL-1β after stimulation with polyinosine-polycytidylic acid than LPS, showing a different preferential response than monocytes. In addition, blockade of nucleotide receptors with oxidized ATP significantly increased human rhinovirus (HRV) recovered 24 hours after infection in BECs, whereas 2′-3′-O-(4-benzoylbenzoyl) ATP treatment of brushed epithelial cells and respiratory cell lines nonsignificantly decreased HRV recovery. IL-1β release was detected after HRV infection in both BECs and brushed cells, but BzATP did not significantly increase IL-1β release further. BEC processing of pro–IL-1β to the mature, cleaved, 17-kD form was confirmed by Western blotting. These results support the expression of functional P2X7 in human lung epithelium, although its role in epithelial pathogen defense is likely independent of IL-1 family cytokine processing.
Keywords: P2X7, IL-1β, human rhinovirus, respiratory epithelium, danger signaling
Clinical Relevance
The nucleotide receptor, P2X7, has been shown to regulate the inflammatory response to a variety of airway pathogens through unclear mechanisms. Our current results show that human, primary respiratory epithelial cells express low levels of functional P2X7 that may contribute in part to control rhinovirus infection. This process is likely independent of mature IL-1β release.
Respiratory epithelium provides a complex physical barrier against foreign material, and is the first site of exposure to pathogens. When infected, respiratory epithelium may serve as a primary site of replication. Once a cell detects an invasion, it may attempt to destroy the pathogen or signal for help to prevent other cells from succumbing to its fate (1). Airway epithelial cells are sources of inflammatory cytokines, including IL-1β and IL-18, as detailed in previously published reviews (2–4), and sometimes generated by unclear mechanisms upon exposure to cigarette smoke (5) or other injury models, including bleomycin (6). Epithelium also responds to a host of other cytokines, including TNF-α and IFN-γ, present during both viral and gram-negative bacterial infections. Efficient release of the IL-1 family of cytokines involves synthesis induced by ligand activation of Toll-like receptors (TLRs) and P2X7-regulated activation of caspase 1, leading to proteolytic cleavage (7). Previous studies in rodent epithelial cells document messenger RNA (mRNA) and protein expression of P2X7, as well as characteristic, nondesensitizing, ATP-induced cation flux, but have not addressed the ability of this receptor to form pores and concomitant capacity for IL-1 family processing.
The IL-1 family of cytokines is important in the pathology of asthma. Airway hyperresponsiveness was reported to be attenuated with IL-1 blockade (8, 9), and subjects with severe asthma demonstrated increased IL-1β in the sputum compared with those with nonsevere asthma (10). IL-33 has been linked to T helper type 2 cytokines, and was reported to have increased expression in severe asthma (11). In addition, IL-18 knockout mice demonstrated decreased inflammation and remodeling during ovalbumin challenge models (12). Because multiple members of the IL-1 family have been shown to be modulated in asthmatic phenotypes, an upstream regulator of IL-1 processing is an attractive investigational tool and target.
ATP has emerged as a mediator of lung inflammation in animal models and clinical studies of patients with asthma, chronic obstructive pulmonary disease (COPD), cystic fibrosis, and idiopathic pulmonary fibrosis (13–16). Extracellular ATP, acting as a danger signal, is released as a consequence of cell damage, stress, infection, allergen, tobacco, or platelet degranulation due to inflammation (17). Despite rapid metabolism, ATP and ADP act on a family of nucleotide receptors expressed by epithelial cells and leukocytes to activate danger-signaling pathways as an adjunct to other cellular responses involved in both the innate and adaptive immune responses (13, 18).
Various P2 purinergic receptors have been implicated in the airway responses to ATP. Respiratory epithelial P2Y2 (a heptohelical, G protein–coupled receptor) has been linked to water efflux and targeted as a potential therapeutic pathway in cystic fibrosis (19). P2Y2 has also been shown to regulate chemotaxis of resident dendritic cells (20). In addition, a P2X7-like nonselective cation channel activity was associated with epithelial cell ciliary beat frequency (21). Genetic deficiency of P2X7 in mice slows the development of ovalbumin-induced inflammation (22), tobacco smoke–triggered emphysema (23), and bleomycin-induced lung fibrosis (6). To balance these findings, we have shown in humans that attenuated P2X7 pore function is a risk factor for virus-induced asthma exacerbations (24), whereas others demonstrated a protective role in eliminating tuberculosis (25), suggesting that this receptor is critical to infection control while also promoting inflammatory conditions. A heterogeneous population of cells is present in the airway both in health and disease. The relative contribution of resident epithelial cells compared with leukocytes in responses to nucleotides in the airway is unresolved.
Given the frequent exposure of bronchial epithelial cells (BECs) to TLR ligands and danger signals, we hypothesized that primary human respiratory epithelium express functional P2X7, and that such expression confers functional processing of IL-1 family cytokines. To begin evaluating the contribution of BEC P2X7 function on the inflammatory response to viral TLR ligand activation or infection, the present study assesses the canonical P2X7 functions in respiratory epithelial cells to extend previous findings regarding channel activity and IL-1 processing observed in cell lines. Herein, we report the function of P2X7 in BECs and a differential response to TLR agonists, including effects upon viral infection.
Materials and Methods
Cell Cultures
Human cells were obtained with approval from the University of Wisconsin–Madison Institutional Review Board. Human primary BECs were isolated from residual tracheal/bronchial rings (26). Subject BECs (B39, B45, and B47) were cultured in supplemented bronchial epithelial growth medium (BEGM; Cambrex, East Rutherford, NJ). Primary epithelial cells from patients with asthma were obtained from brushings during bronchoscopy, as previously described (27). Additional details are in the Materials and Methods in the online supplement.
Fluorescent Dye Uptake
BECs (5 × 104/chamber) on collagen-IV (Sigma, St. Louis, MO) –coated chamber slides (Thermo Fisher Scientific, Pittsburgh, PA) were cultured in BEGM for 24 hours, washed with Hepes-buffered saline (HBS; 130 mM NaCl, 5 mM KCl, 20 mM Hepes, 0.1% BSA, 10 mM glucose, pH 7.4) and stimulated with HBS containing 10 μM YO-PRO-1 (Invitrogen, Carlsbad, CA) with or without 300 μM 2′-3′-O-(4-benzoylbenzoyl) ATP (BzATP; Sigma) at 37°C for 20 minutes. Cells were washed three times with HBS before observation by fluorescent microscopy (Olympus, Minneapolis, MN).
Immunofluorescense
Cells were fixed and permeablized by standard methods before incubation with anti-P2X7 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) followed by Alexa donkey anti-rabbit antibody (Invitrogen) and 4′,6-diamidino-2-phenylindole (Invitrogen). Details are in the Materials and Methods in the online supplement.
Cell Stimulation
Polyinosine-polycytidylic acid, LPS, ATP.
BECs (1.5 × 105/well) were seeded on collagen-IV–coated 12-well plates (Costar, Cambridge, MA), cultured overnight in BEGM, followed by 24 hours in BEGM with vehicle, 3 μg/ml polyinosine-polycytidylic acid (Poly (I:C); Sigma) or 10 μg/ml LPS (Sigma). Cells were washed with HBS and stimulated 30 minutes with 3 mM ATP (Sigma) or vehicle in HBS.
IFN-γ/TNF-α.
BECs (2 × 105/well) were seeded onto collagen-IV–coated six-well plates (Costar) and cultured 24 hours in BEGM without hydrocortisone supplementation before stimulation with 10 ng/ml IFN-γ (Peprotech, Rocky Hill, NJ) and 5 ng/ml TNF-α (BD Pharmingen, San Diego, CA) for 48 hours.
IL-1 receptor antagonist and cell-derived IL-1β.
A549 cells were incubated for 24 hours with media-diluted BEC supernatants and IL-1 receptor antagonist (Sigma). Details similar to those of previous studies (28) are in the Materials and Methods in the online supplement.
Protein Determination
ELISA.
ELISAs were performed as previously described (29).
Immunoblotting.
Details are in the Materials and Methods in the online supplement.
RNA Isolation and PCR Techniques
Standard methods were used and can be found in the online supplement.
Human Rhinovirus Serotype 1a Infection
Purified and concentrated human rhinovirus serotype 1a (HRV1a) was provided by Dr. W. M. Lee (University of Wisconsin–Madison). BECs (1.5 × 105/well) were seeded in 12-well plates for 24 hours and pretreated with 500 μM periodate oxidized ATP (oATP; Sigma) or vehicle in BEGM for 2 hours at 37°C. After PBS washing, BECs were infected with 350 μl/well of HRV1a diluted in reduced hydrocortisone (10−8 M) BEGM to 10 PFU/cell with or without 100 μM BzATP, or incubated with media alone for 2 hours at 34°C. After three PBS washes, 1 ml BEGM with or without 100 μM BzATP was added to each well and incubated 24 hours at 34°C.
Statistical Analysis
Cytokines and HRV were normalized by natural log transformations for statistical analyses. Two-tailed t tests, one-way ANOVA, and repeated measures ANOVA tests were made with SigmaPlot (Systat Software Inc., San Jose, CA) (significance level of P = 0.05). Pair-wise comparisons were Bonferroni corrected.
Results
Donor BECs Exhibit Modest BzATP-Induced Dye Uptake
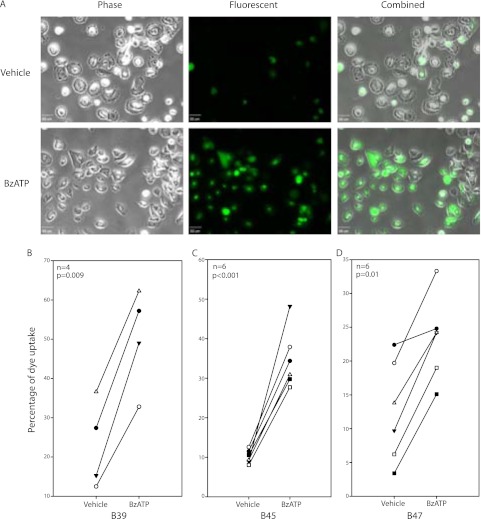
A characteristic of P2X7 is its agonist-induced ability to form a nonselective cation pore in the plasma membrane. BzATP activates P2X7 at lower concentrations than is required for activation of other purinergic receptors (30), and YO-PRO-1, a 629-Da intercalating fluorescent dye, will move through these open pores specific to P2X7 activation. Initial BEC P2X7 stimulation experiments using a plate reader suggested slower and less robust YO-PRO-1 uptake than monocytic controls (data not shown). This observation led us to adopt a method of observing cell dye uptake via fluorescent microscopy. After stimulation with BzATP, monolayer BECs (B39, B45, B47) with intracellular YO-PRO-1 were counted and reported as a proportion of all cells. Representative images for B45 are shown in Figure 1A. The percentage of BzATP-stimulated dye uptake was significantly increased in all BEC groups (Figures 1B–1D), with an average fold increase of positive cells over vehicle of 2.2, 3.4, and 1.9 in B39 (n = 4), B45 (n = 6), and B47 (n = 6) BECs, respectively.
Figure 1.
Bronchial epithelial cells (BECs) have functional P2X7 pore activity. Fluorescent dye, YO-PRO-1, uptake in BECs after 2′-3′-O-(4-benzoylbenzoyl) ATP (BzATP) stimulation was observed by fluorescent microscopy and counted as a proportion of all cells. Representative images of BEC B45 are shown (A). The percentage of adherent BECs containing YO-PRO-1 was calculated and BzATP-treated cells demonstrated a significant increase of uptake for all three BECs (B_–_D). Unpaired t tests were performed within each group. Scale bar, 50 μm.
P2X7 Is Expressed in BECs and Enhanced by Inflammatory Cytokine Priming
P2X7 mRNA was observed in all three BECs examined, with a RT-PCR product the same size as that from human embryonic kidney (HEK)-293 cells transfected with human P2X7 (see Figure E1A in the online supplement). The truncated P2X7 splice variant (P2X7-j), reported to act as a dominant negative (31), was not observed in any monolayer BECs (data not shown) using standard RT-PCR. As recent reports indicate that P2X7 may form a large pore complex via association with the hemichannel protein, Pannexin-1 (32), analysis of Pannexin-1 expression by RT-PCR also revealed a specific band in all three subject BECs (Figure E1B).
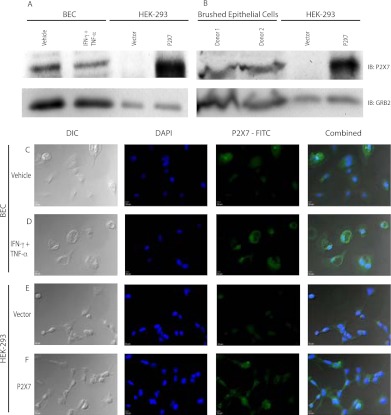
BEC lysates were probed for P2X7 by Western blotting with positive controls, including THP-1 cells differentiated for 2 days with 100 nM phorbol-12-myristate-13-acetate (PMA; Sigma) and stably transfected P2X7 HEK-293 cells. A 72-kD band was observed in both positive controls, consistent with the size of P2X7, whereas bands were difficult to observe at 72 kD in any BECs. Large amounts of cell lysate were required for detection of P2X7 in BECs and brushed epithelial cells compared with other cell types (Figures 2A and 2B).
Figure 2.
P2X7 is detected in primary respiratory epithelial cells. BECs cultured with vehicle or TNF-α/IFN-γ for 24 hours (A) and brushed epithelial cells from two donors (B) were probed for P2X7 by Western blotting, and indicate GRB2 (growth factor receptor-bound protein 2) as a loading control. Human embryonic kidney (HEK)-293 cells with vector or P2X7 were used as controls. BECs treated with vehicle (C) or TNF-α/IFN-γ (D) for 48 hours were examined for P2X7 protein. DAPI (4′,6-diamidino-2-phenylindole) and secondary antibody were coincubated before visualization. HEK-293 cells contain an empty vector (E) or express P2X7 (F) for negative and positive controls, respectively. Differential interference contrast and fluorescent signals are combined in the right panels. Scale bar, 10 μm.
Previously, Humphreys and Dubyak (7) showed that TNF-α/IFN-γ were the most potent combination of proinflammatory cytokines tested to increase P2RX7 expression in THP-1 cells. Monolayer BECs stimulated with TNF-α and IFN-γ for 48 hours were used for quantitative RT-PCR measures of genes related to P2X7 pore function. Compared with unstimulated cells, incubation with combined TNF-α/IFN-γ synergistically increased expression of P2X7 mRNA and more modestly increased Pannexin-1 mRNA (Table 1). The P2X7-j splice variant was not detected in any BEC, even with cytokine priming. BECs B39 (n = 1) and B45 (n = 2) were used to examine immunofluorescent localization of P2X7; priming of these BECs with TNF-α and IFN-γ increased immunofluorescent signal for P2X7. HEK-293 cells with P2X7 and empty pcDNA3 vectors were used as controls. Representative images of BEC B45 are shown in Figures 2C–2F.
TABLE 1.
INFLAMMATORY CYTOKINES INCREASE P2X7 MESSENGER RNA
| P2X7 | Pannexin-1 | |
|---|---|---|
| B39 | 4.36 (2.22) | 1.78 (0.33) |
| B45 | 7.82 (4.72) | 2.11 (0.29) |
| B47 | 18.69 (13.62) | 2.10 (0.31) |
ATP Promotes IL-1β Release in Poly (I:C)–Primed BECs
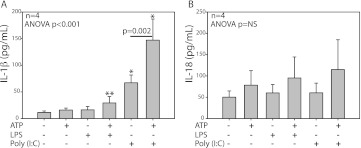
Reports indicate that P2X7 receptors modulate early IL-1β, and, to a lesser extent, IL-18 processing and release in response to ATP in LPS-primed monocytes (33). In fact, P2X7 activation of caspase-1 has been reported to require TLR engagement (34). Intestinal epithelial cells express IL-1β and display enhanced expression after LPS exposure (35). However, it is not clear if ATP-induced release of IL-1β occurs in LPS-primed human BECs, or whether other TLR receptors can also induce IL-1β release in BECs. To address these questions, either LPS or Poly (I:C) was used to prime monolayer B45 BECs for 24 hours. Poly (I:C) priming induced the release of IL-1β, which was augmented by ATP treatment (n = 4; Figure 3A). However, IL-18 did not display a similar increase in rapid release after Poly (I:C) priming (n = 4; Figure 3B). Priming with Poly (I:C) in respiratory epithelium appears similar to LPS priming in THP-1 monocytes as separate systems to augment release of IL-1β upon ATP stimulation (Figure E2A). That the methods of isolating BECs results in a cell population with greater than 99% purity (36), combined with the differential sensitivity of BECs to Poly (I:C) priming, is enhanced compared with LPS priming is supportive and consistent with a nonleukocyte cell population releasing the IL-1β measured in these experiments.
Figure 3.
ATP increases IL-1β release from polyinosine-polycytidylic acid (Poly (I:C))–primed BECs. Supernatants from BEC B45 (n = 4) primed with LPS or Poly (I:C) were collected after stimulation with ATP for 30 minutes and analyzed by ELISA. IL-1β levels were augmented by Poly (I:C) priming, which was further increased with ATP treatment (A). IL-18 did not demonstrate the same pattern of release (B). The limit of detection for both cytokines was 12.5 pg/ml and data are shown as mean ± SD. Pairwise comparisons were Bonferroni corrected. *P < 0.05 compared with vehicle-, ATP-, LPS-, and ATP/LPS-treated cells. **P < 0.05 compared with vehicle.
HRV-Stimulated IL-1β Release Is Largely Independent of P2X7, Despite Limited Differences in HRV Infection after Nucleotide Receptor Modulation
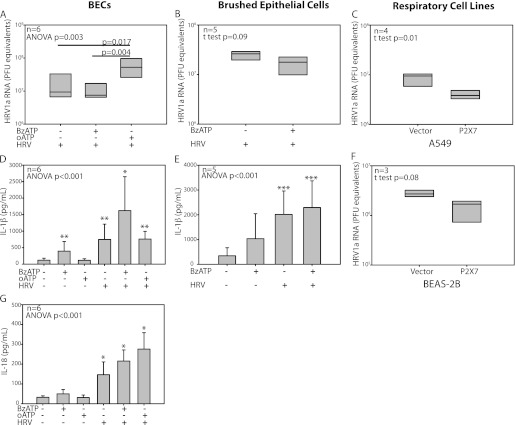
BECs showed an increased pro–IL-1β after infection with HRV1a (Figure E2B). BECs cotreated with BzATP during and after HRV infection demonstrated a small, nonsignificant decrease in HRV load at 24 hours. However, a more robust, significant difference in HRV recovery was observed when comparing either vehicle- or BzATP-treated cells with oATP-treated cells (n = 6; Figure 4A). The magnitude of the difference was approximately a half-log increase in recovered HRV1a with oATP treatment, although the amount of virus associated with the BECs was not different at the end of the 2-hour infection period (data not shown). Brushed primary respiratory epithelial cells from bronchoscopy also displayed a trend to decreased HRV production with BzATP stimulation (n = 5; Figure 4B).
Figure 4.
Nucleotide receptor blockade increases HRV recovery after infection, whereas HRV infection increases IL-1 family cytokine release. Cellular HRV levels at 24 hours were affected by nucleotide receptor modulators. Oxidized ATP (oATP) treatment of BECs increased HRV1a compared with both vehicle and BzATP groups (A), whereas brushed epithelial cells trended to a small decrease with BzATP treatment (B). Respiratory cell lines A549 (C) and BEAS-2B (F) transfected with either vector control or P2X7 were also infected with HRV1a for 24 hours. Supernatants of BECs or brushed epithelial cells treated with oATP and/or BzATP and incubated with HRV1a were analyzed for cytokine production. BEC IL-1β measured at 24 hours (D) was increased with HRV infection in all groups compared with vehicle or oATP treated. Epithelial cells from bronchoscopic brushings showed similar results with IL-1β (E), generating more IL-1β in the supernatant at 24 hours compared with vehicle, but not BzATP-treated cells. BEC IL-18 measured at 24 hours (G) was increased in all HRV groups. Pairwise comparisons are Bonferroni corrected. For cytokine measures: *P < 0.05 compared with vehicle-, BzATP-, and oATP-treated cells; **P < 0.05 compared with vehicle- and oATP-treated cells; ***P < 0.05 compared with vehicle. Box plots display median and interquartile range for A–C and F, and bar graphs show mean ± SD for D, E, and G.
We performed similar HRV infection experiments in human respiratory cell lines to confirm the observation that blocked P2X7 function increased HRV recovery. Both A549 and BEAS-2B cell lines had undetectable P2X7 when measured by quantitative RT-PCR, leading us to generate stably transfected cells containing either vector control or P2X7 constructs. HRV1a infection of these cell lines was consistent with the above results, demonstrating an increased level of HRV1a in the respiratory cells without P2X7 at 24 hours after infection (Figures 4C and 4F).
HRV infection increased IL-1β release 24 hours after monolayer BEC infection with HRV1a. Cotreatment of BECs with BzATP during infection augmented the release of IL-1β from infected cells compared with BzATP treatment alone (n = 6). The measured IL-1β and IL-18 levels in BEC supernatants are shown in Figures 4D and 4G. Although results are similar to the early release observed with Poly (I:C) priming, the HRV infection appears to drive cytokine generation in these experiments at 24 hours. Epithelial cells obtained by bronchoscopic brushings (n = 5) infected with HRV1a showed similar trends in IL-1β production as that in BECs (Figure 4E). IL-1β was undetectable in A549 and BEAS-2B cell lines, with and without P2X7, whether or not infected with HRV.
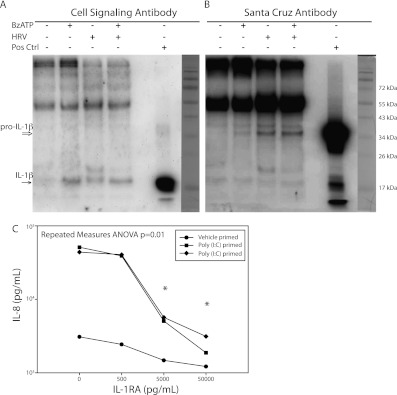
To confirm that the IL-1β detected by ELISA was not merely nonselective release of pro–IL-1β induced by TLR activation, Western blotting was performed on cell-free supernatants. Bands were detectable near the 17-kD expected size with varying efficiency with two separate antibodies (Figures 5A and 5B), as well as bands consistent with pro–IL-1β in HRV-infected sample supernatants. Lanes with detectable bands representing cleaved IL-1β corresponded to the conditions in Figure 4, showing an increased level of IL-1β measured by ELISA when compared with vehicle-treated cells. In addition, when using HRV1a preparations at 20 times the amount used in any experiments, IL-1β was below the limit of detection measured by ELISA.
Figure 5.
IL-1β released from BECs is cleaved and demonstrates functional activity. Western blotting of concentrated supernatants from BEC B45 infected for 24 hours with HRV1a and/or stimulated with BzATP is shown with detectable bands similar in size to the positive control 17-kD band from primed THP-1 cells, and is representative of three experiments. Immunoblots with Cell Signaling (Danvers, MA) (A) and Santa Cruz Biotechnology (Santa Cruz, CA) (B) antibodies display preferential detection of the cleaved and pro forms of IL-1β, respectively. Positive control indicates LPS-primed and ATP-treated THP-1 cells. Molecular weight markers are shown on the right. (C) Supernatants from BECs primed with Poly (I:C) or vehicle and treated with ATP were used to culture A549 cells for 24 hours. Increasing concentrations of IL-1 receptor antagonist (IL-1RA) were added and IL-8 was measured by ELISA. *P < 0.05 compared with IL-8 release with no IL-1RA. Data points are the average of technical duplicates.
Culture with BEC Supernatants Demonstrates IL-1β–Induced Release of IL-8
BEC IL-8 concentrations in the same supernatants as those in Figure 4D correlated to IL-1β measurements (natural log normalized, Pearson R = 0.84; P = 0.04). As further evidence for a functional role for released IL-1β, cell supernatants from Poly (I:C)–primed and ATP-treated BECs elicited a robust production of IL-8 from A549 cells similar to previous studies (28). The IL-8 production was significantly decreased by IL-1 receptor antagonist in a dose-dependent manner (Figure 5C).
Discussion
Whereas P2X7 function in leukocytes is reasonably well established, its role in epithelial cell defenses is less understood. We provide additional evidence for P2X7 function in human respiratory epithelium, including pore activity, a property associated with active P2X7. To our knowledge, the incorporation of YO-PRO-1 into BECs by BzATP stimulation described here is the first report of P2X7 pore function in primary human respiratory epithelium. Recent reports indicate that Pannexin-1, in physical association with the P2X7 receptor, is linked to both dye uptake and IL-1β release (32, 37, 38). Our results confirm that primary BECs also express Pannexin-1 mRNA, and this is supported by our observation of pore activity in epithelial cells. Kim and colleagues (39) previously reported that human nasal epithelial cells express P2X7 mRNA, but did not show protein expression or pore activity. Our results reinforce those from animal models, where studies indicate airway epithelial cells express P2X7 mRNA, protein, and channel activity (40–42). In addition, the ATP-initiated increase in rapid BEC IL-1 cytokine release was augmented by TLR3 engagement. Whether the detected IL-1β is immature or cleaved and provides adequate biological activity in vivo is often assumed in the literature, whereas others, including Riteau and colleagues (6), have demonstrated processing capability in immortalized respiratory cells (BEAS-2B) under various stimuli. We demonstrate comparable results in primary respiratory cells, as well as in response to viral infection and TLR agonists. Although we cannot rule out some contribution of pro–IL-1β measured by ELISA, we have confirmed by Western analysis that at least some portion of the IL-1β present is cleaved upon P2X7 stimulation in human respiratory epithelium, and have demonstrated that, under these conditions, the released IL-1β confers subsequent production and release of IL-8. In total, we have strengthened the evidence for the presence of functional P2X7 in primary human respiratory cells.
HRV, a prominent respiratory pathogen, augmented the release of IL-1β in primary epithelial cells. Many different pathways, including nonclassical routes (43), for the maturation and release of the IL-1 family of cytokines have been described, and the conditions under which P2X7 mediates IL-1β release in respiratory epithelial cells is becoming more clear. However, our results add to the debate of what cellular machinery and at which stage of processing IL-1β may be released from respiratory epithelium under different conditions. LPS recognition requires the functional interaction of TLR4 with MD-2 and CD14 (44). Although airway epithelial cells express most TLRs (45), TLR4 appears to have a limited role (46), in agreement with our finding of less IL-1β induction from LPS compared with Poly (I:C), unlike monocytic lineage cells. Our early detection of IL-1β after Poly (I:C) stimulation in BECs is in contrast to experiments in peripheral blood mononuclear cells (47). In addition, it is possible that pro–IL-1β may be cleaved after cytolysis. An intriguing possibility is whether individuals with known loss-of-function P2X7 alleles would generate less active IL-1β during infection, although there was not a significant difference at 24 hours in our experiments with oATP treatment. Rapid release (30 min) of IL-1β in monocytes and macrophages and matrix metalloproteinase-9 release in monocytes are all dependent upon P2X7, whereas detection of IL-1β at later time points (24 h) may be observed with non–P2X7-dependent pathways in monocytes, but not macrophages (47–49). In addition, other experimental systems markers, including IL-10 and TNF-α, have shown differences upon LPS whole-blood stimulation at both 6 and 24 hours, secondary to differences in functional P2X7 (50). Clearly, the timing of assessment of mediator release, as well as assessment of cell population, may be critical to the discernment of P2X7-dependent effects.
P2X7 is a known modulator of intracellular infection control. In fact, P2X7 function is prominently involved in the control of tuberculosis. IL-1β and P2X7 activation are linked to neutrophil influx at areas of injury and inflammation (51), and we have previously demonstrated a correlation between P2X7 pore activity and nasal neutrophil levels during naturally acquired colds (24). In the experiments performed here, blockade of purine signaling during infection of BECs increased HRV1a levels, even with no leukocyte contribution to the experimental system. These initial findings with HRV support our previous report that a decrease in P2X7 function predisposes to the risk of asthma exacerbations after natural HRV infections (24). Given that inflammatory cytokines enhance expression of P2X7 in BECs, it is possible that there may be a paracrine effect to limit spread of the infection. TLR priming also increases resistance to HRV infection, and, in subjects with asthma, HRV infection is patchy, providing a way for P2X7 to act in a paracrine fashion to limit viral spread. Extending the in vitro epithelial cell experiments to air–liquid interface differentiated cells would be a natural extension of our monolayer system to investigate these processes and what role P2X7 plays in infectious resistance by differentiated epithelium.
When combined with recent reports (6, 22, 52), a growing role for purinergic receptors in general, and P2X7 in particular, within lung disease is taking shape. Our results support the presence and function of P2X7 in human lung epithelia, independent of leukocytes with an antiviral capacity, including IL-1β processing and release. Finally, determining the full extent of P2X7 function in primed and differentiated cells will illuminate the therapeutic potential of receptor modulation in disease states, including asthma.
Supplementary Material
Disclosures
Online Supplement
Acknowledgments
The authors thank Dr. James Gern and Ms. Becky Brockman-Schneider for assistance with human bronchial epithelial cells, Dr. Wai-Ming Lee for human rhinovirus (HRV) generation, Dr. Yury Bochkov for HRV infection advice, and Dr. Nizar Jarjour for support and subject recruitment for brushed epithelial cells.
Footnotes
This work was supported by American Thoracic Society grant 06-017, and by National Institutes of Health (NIH) grants K23 HL081492, P01 HL088594, and 9U54TR000021.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org
References
- 1.Leslie M. Cell biology: internal affairs. Science 2009;326:929–931 [DOI] [PubMed] [Google Scholar]
- 2.Hammad H, Lambrecht BN. Dendritic cells and epithelial cells: linking innate and adaptive immunity in asthma. Nat Rev Immunol 2008;8:193–204 [DOI] [PubMed] [Google Scholar]
- 3.Kato A, Schleimer RP. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol 2007;19:711–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stadnyk AW. Cytokine production by epithelial cells. FASEB J 1994;8:1041–1047 [DOI] [PubMed] [Google Scholar]
- 5.Rusznak C, Sapsford RJ, Devalia JL, Shah SS, Hewitt EL, Lamont AG, Davies RJ, Lozewicz S. Interaction of cigarette smoke and house dust mite allergens on inflammatory mediator release from primary cultures of human bronchial epithelial cells. Clin Exp Allergy 2001;31:226–238 [DOI] [PubMed] [Google Scholar]
- 6.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, Kanellopoulos J, Quesniaux VF, Marchand-Adam S, Crestani B, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med 2010;182:774–783 [DOI] [PubMed] [Google Scholar]
- 7.Humphreys BD, Dubyak GR. Modulation of P2X7 nucleotide receptor expression by pro- and anti-inflammatory stimuli in THP-1 monocytes. J Leukoc Biol 1998;64:265–273 [DOI] [PubMed] [Google Scholar]
- 8.Johnson VJ, Yucesoy B, Luster MI. Prevention of IL-1 signaling attenuates airway hyperresponsiveness and inflammation in a murine model of toluene diisocyanate–induced asthma. J Allergy Clin Immunol 2005;116:851–858 [DOI] [PubMed] [Google Scholar]
- 9.Nakae S, Komiyama Y, Yokoyama H, Nambu A, Umeda M, Iwase M, Homma I, Sudo K, Horai R, Asano M, et al. IL-1 is required for allergen-specific Th2 cell activation and the development of airway hypersensitivity response. Int Immunol 2003;15:483–490 [DOI] [PubMed] [Google Scholar]
- 10.Hastie AT, Moore WC, Meyers DA, Vestal PL, Li H, Peters SP, Bleecker ER. Analyses of asthma severity phenotypes and inflammatory proteins in subjects stratified by sputum granulocytes. J Allergy Clin Immunol 2010;125:1028–1036e1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prefontaine D, Lajoie-Kadoch S, Foley S, Audusseau S, Olivenstein R, Halayko AJ, Lemiere C, Martin JG, Hamid Q. Increased expression of IL-33 in severe asthma: evidence of expression by airway smooth muscle cells. J Immunol 2009;183:5094–5103 [DOI] [PubMed] [Google Scholar]
- 12.Yamagata S, Tomita K, Sato R, Niwa A, Higashino H, Tohda Y. Interleukin-18–deficient mice exhibit diminished chronic inflammation and airway remodelling in ovalbumin-induced asthma model. Clin Exp Immunol 2008;154:295–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MA, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, et al. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 2007;13:913–919 [DOI] [PubMed] [Google Scholar]
- 14.Lommatzsch M, Cicko S, Muller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Durk T, Zissel G, Ferrari D, et al. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010;181:928–934 [DOI] [PubMed] [Google Scholar]
- 15.Gabriel SE, Makhlina M, Martsen E, Thomas EJ, Lethem MI, Boucher RC. Permeabilization via the P2X7 purinoreceptor reveals the presence of a Ca2+-activated Cl− conductance in the apical membrane of murine tracheal epithelial cells. J Biol Chem 2000;275:35028–35033 [DOI] [PubMed] [Google Scholar]
- 16.Mortaz E, Braber S, Nazary M, Givi ME, Nijkamp FP, Folkerts G. ATP in the pathogenesis of lung emphysema. Eur J Pharmacol 2010;619:92–96 [DOI] [PubMed] [Google Scholar]
- 17.Willart MA, Lambrecht BN. The danger within: endogenous danger signals, atopy and asthma. Clin Exp Allergy 2009;39:12–19 [DOI] [PubMed] [Google Scholar]
- 18.Myrtek D, Idzko M. Chemotactic activity of extracellular nucleotideson human immune cells. Purinergic Signal 2007;3:5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Accurso FJ, Moss RB, Wilmott RW, Anbar RD, Schaberg AE, Durham TA, Ramsey BW. Denufosol tetrasodium in patients with cystic fibrosis and normal to mildly impaired lung function. Am J Respir Crit Care Med 2011;183:627–634 [DOI] [PubMed] [Google Scholar]
- 20.Muller T, Robaye B, Vieira RP, Ferrari D, Grimm M, Jakob T, Martin SF, Di Virgilio F, Boeynaems JM, Virchow JC, et al. The purinergic receptor P2Y2 receptor mediates chemotaxis of dendritic cells and eosinophils in allergic lung inflammation. Allergy 2010;65:1545–1553 [DOI] [PubMed] [Google Scholar]
- 21.Taylor AL, Schwiebert LM, Smith JJ, King C, Jones JR, Sorscher EJ, Schwiebert EM. Epithelial P2X purinergic receptor channel expression and function. J Clin Invest 1999;104:875–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Müller T, Paula Vieira R, Grimm M, Dürk T, Cicko S, Zeiser R, Jakob T, Martin SF, Blumenthal B, Sorichter S, et al. A potential role for P2X7R in allergic airway inflammation in mice and humans. Am J Respir Cell Mol Biol 2011;44:456–464 [DOI] [PubMed] [Google Scholar]
- 23.Lucattelli M, Cicko S, Muller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Durk T, et al. P2X7 receptor signaling in the pathogenesis of smoke-induced lung inflammation and emphysema. Am J Respir Cell Mol Biol 2011;44:423–429 [DOI] [PubMed] [Google Scholar]
- 24.Denlinger LC, Shi L, Guadarrama A, Schell K, Green D, Morrin A, Hogan K, Sorkness RL, Busse WW, Gern JE. Attenuated P2X7 pore function as a risk factor for virus-induced loss of asthma control. Am J Respir Crit Care Med 2009;179:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saunders BM, Fernando SL, Sluyter R, Britton WJ, Wiley JS. A loss-of-function polymorphism in the human P2X7 receptor abolishes ATP-mediated killing of mycobacteria. J Immunol 2003;171:5442–5446 [DOI] [PubMed] [Google Scholar]
- 26.Jakiela B, Brockman-Schneider R, Amineva S, Lee WM, Gern JE. Basal cells of differentiated bronchial epithelium are more susceptible to rhinovirus infection. Am J Respir Cell Mol Biol 2008;38:517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bochkov YA, Hanson KM, Keles S, Brockman-Schneider RA, Jarjour NN, Gern JE. Rhinovirus-induced modulation of gene expression in bronchial epithelial cells from subjects with asthma. Mucosal Immunol 2010;3:69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coulter KR, Wewers MD, Lowe MP, Knoell DL. Extracellular regulation of interleukin (IL)-1beta through lung epithelial cells and defective IL-1 type II receptor expression. Am J Respir Cell Mol Biol 1999;20:964–975 [DOI] [PubMed] [Google Scholar]
- 29.Kelly EA, Rodriguez RR, Busse WW, Jarjour NN. The effect of segmental bronchoprovocation with allergen on airway lymphocyte function. Am J Respir Crit Care Med 1997;156:1421–1428 [DOI] [PubMed] [Google Scholar]
- 30.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol 2000;40:563–580 [DOI] [PubMed] [Google Scholar]
- 31.Feng YH, Li X, Wang L, Zhou L, Gorodeski GI. A truncated P2X7 receptor variant (P2X7-j) endogenously expressed in cervical cancer cells antagonizes the full-length P2X7 receptor through hetero-oligomerization. J Biol Chem 2006;281:17228–17237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J 2006;25:5071–5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1beta and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem 2001;276:3820–3826 [DOI] [PubMed] [Google Scholar]
- 34.Kahlenberg JM, Lundberg KC, Kertesy SB, Qu Y, Dubyak GR. Potentiation of caspase-1 activation by the P2X7 receptor is dependent on TLR signals and requires NF-kappaB–driven protein synthesis. J Immunol 2005;175:7611–7622 [DOI] [PubMed] [Google Scholar]
- 35.Waterhouse CC, Stadnyk AW. Rapid expression of IL-1beta by intestinal epithelial cells in vitro. Cell Immunol 1999;193:1–8 [DOI] [PubMed] [Google Scholar]
- 36.Schroth MK, Grimm E, Frindt P, Galagan DM, Konno SI, Love R, Gern JE. Rhinovirus replication causes RANTES production in primary bronchial epithelial cells. Am J Respir Cell Mol Biol 1999;20:1220–1228 [DOI] [PubMed] [Google Scholar]
- 37.Pelegrin P, Surprenant A. Dynamics of macrophage polarization reveal new mechanism to inhibit IL-1beta release through pyrophosphates. EMBO J 2009;28:2114–2127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, Vandenabeele P, Nunez G. Pannexin-1–mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 2007;26:433–443 [DOI] [PubMed] [Google Scholar]
- 39.Kim CH, Kim SS, Choi JY, Shin JH, Kim JY, Namkung W, Lee JG, Lee MG, Yoon JH. Membrane-specific expression of functional purinergic receptors in normal human nasal epithelial cells. Am J Physiol Lung Cell Mol Physiol 2004;287:L835–L842 [DOI] [PubMed] [Google Scholar]
- 40.Marino A, Rodrig Y, Metioui M, Lagneaux L, Alzola E, Fernandez M, Fogarty DJ, Matute C, Moran A, Dehaye JP. Regulation by P2 agonists of the intracellular calcium concentration in epithelial cells freshly isolated from rat trachea. Biochim Biophys Acta 1999;1439:395–405 [DOI] [PubMed] [Google Scholar]
- 41.Chen Z, Jin N, Narasaraju T, Chen J, McFarland LR, Scott M, Liu L. Identification of two novel markers for alveolar epithelial type I and II cells. Biochem Biophys Res Commun 2004;319:774–780 [DOI] [PubMed] [Google Scholar]
- 42.Ma W, Korngreen A, Weil S, Cohen EB, Priel A, Kuzin L, Silberberg SD. Pore properties and pharmacological features of the P2X receptor channel in airway ciliated cells. J Physiol 2006;571:503–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qu Y, Franchi L, Nunez G, Dubyak GR. Nonclassical IL-1 beta secretion stimulated by P2X7 receptors is dependent on inflammasome activation and correlated with exosome release in murine macrophages. J Immunol 2007;179:1913–1925 [DOI] [PubMed] [Google Scholar]
- 44.Lee HK, Dunzendorfer S, Tobias PS. Cytoplasmic domain–mediated dimerizations of Toll-like receptor 4 observed by beta-lactamase enzyme fragment complementation. J Biol Chem 2004;279:10564–10574 [DOI] [PubMed] [Google Scholar]
- 45.Melkamu T, Squillace D, Kita H, O'Grady SM. Regulation of TLR2 expression and function in human airway epithelial cells. J Membr Biol 2009;229:101–113 [DOI] [PubMed] [Google Scholar]
- 46.Muir A, Soong G, Sokol S, Reddy B, Gomez MI, Van Heeckeren A, Prince A. Toll-like receptors in normal and cystic fibrosis airway epithelial cells. Am J Respir Cell Mol Biol 2004;30:777–783 [DOI] [PubMed] [Google Scholar]
- 47.Ward JR, West PW, Ariaans MP, Parker LC, Francis SE, Crossman DC, Sabroe I, Wilson HL. Temporal interleukin-1beta secretion from primary human peripheral blood monocytes by P2X7-independent and P2X7-dependent mechanisms. J Biol Chem 2010;285:23147–23158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gu BJ, Wiley JS. Rapid ATP-induced release of matrix metalloproteinase 9 is mediated by the P2X7 receptor. Blood 2006;107:4946–4953 [DOI] [PubMed] [Google Scholar]
- 49.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. Il-1beta processing in host defense: beyond the inflammasomes. PLoS Pathog 2010;6:e1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Denlinger LC, Angelini G, Schell K, Green DN, Guadarrama AG, Prabhu U, Coursin DB, Bertics PJ, Hogan K. Detection of human P2X7 nucleotide receptor polymorphisms by a novel monocyte pore assay predictive of alterations in lipopolysaccharide-induced cytokine production. J Immunol 2005;174:4424–4431 [DOI] [PubMed] [Google Scholar]
- 51.McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 2010;330:362–366 [DOI] [PubMed] [Google Scholar]
- 52.Cicko S, Lucattelli M, Muller T, Lommatzsch M, De Cunto G, Cardini S, Sundas W, Grimm M, Zeiser R, Durk T, et al. Purinergic receptor inhibition prevents the development of smoke-induced lung injury and emphysema. J Immunol 2010;185:688–697 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Disclosures
Online Supplement