Regulated membrane protein entry into flagella is facilitated by cytoplasmic microtubules and does not require IFT (original) (raw)
. Author manuscript; available in PMC: 2014 Aug 5.
Published in final edited form as: Curr Biol. 2013 Jul 25;23(15):1460–1465. doi: 10.1016/j.cub.2013.06.025
SUMMARY
The membrane protein composition of the primary cilium, a key sensory organelle, is dynamically regulated during cilium-generated signaling [1, 2]. During ciliogenesis, ciliary membrane proteins, along with structural and signaling proteins, are carried through the multi-component, intensely studied ciliary diffusion barrier at the base of the organelle [3–8] by intraflagellar transport (IFT) [9–18]. A favored model is that signaling-triggered accumulation of previously excluded membrane proteins in fully formed cilia [19–21] also requires IFT, but direct evidence is lacking. Here, in studies of regulated entry of a membrane protein into the flagellum of Chlamydomonas, we show that cells use an IFT-independent mechanism to breach the diffusion barrier at the flagellar base. In resting cells, a flagellar signaling component [22], the integral membrane polypeptide SAG1-C65, is uniformly distributed over the plasma membrane and excluded from the flagellar membrane. Flagellar adhesion-induced signaling triggers rapid, striking redistribution of the protein to the apical ends of the cells concomitantly with entry into the flagella. Protein polarization and flagellar enrichment are facilitated by cytoplasmic microtubules. Using a conditional anterograde IFT mutant, we demonstrate that the IFT machinery is not required for regulated SAG1-C65 entry into flagella. Thus, integral membrane proteins can negotiate passage through the ciliary diffusion barrier without the need for a motor.
RESULTS
SAG1-HA is localized primarily on the plasma membrane on the cell body
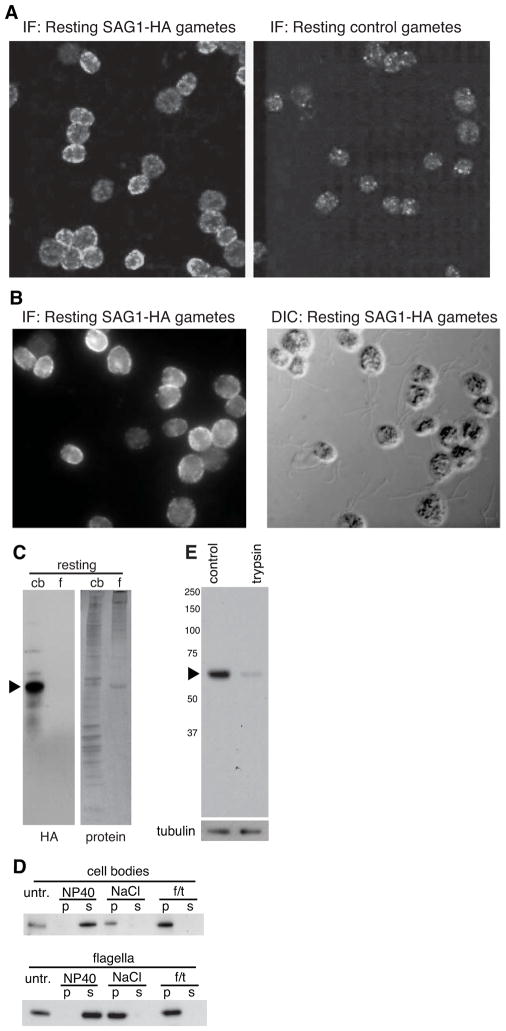
When Chlamydomonas plus and minus gametes are mixed together, they adhere to each other by interaction of the plus agglutinin peripheral membrane polypeptide encoded by the SAG1 gene with the minus agglutinin encoded by the SAD1 gene [22]. Flagellar adhesion engages the intraflagellar transport machinery [23, 24] to activate a protein kinase-dependent signaling pathway within the flagella that rapidly leads to a 10–20 fold increase in intracellular cAMP levels, thereby triggering gamete activation in preparation for cell-cell fusion [22]. Much earlier studies indicated that cells possessed a pool [25] of inactive agglutinins on the cell body [26–29] that was recruited to flagella during flagellar adhesion-induced signaling [23, 29], but the results were difficult to interpret because a direct probe was unavailable. To study potential, signaling-regulated entry of a membrane protein into flagella, we expressed a SAG1 transgene encoding a C-terminal HA tag in the flagellar adhesion mutant sag1-5 [30] (Supplemental Figures S1A – S1C). Immunofluorescence studies demonstrated that, whereas control gametes lacked anti-HA-staining (Figure 1A, right panel), the SAG1-HA/sag1-5 gametes (hereafter termed SAG1-HA gametes) stained strongly (Figures 1A and 1B, left panels). HA staining was abundant on the plasma membrane of cell bodies, with little if any on flagella. The HA signal, portions of which appeared as puncta, was uniformly distributed on the periphery of the cell body (ring-staining), indicating that it was associated with the plasma membrane (Figures 1A and 1B, left panels; Supplemental Movie S1). These results indicated that SAG1-HA cells expressed an HA-tagged polypeptide encoded by the SAG1-HA transgene that localized on the plasma membrane and was mostly excluded from the flagellar membrane.
Figure 1. SAG1-HA is evenly distributed in the cell body plasma membrane and is expressed in gametes primarily as a 65 kDa C-terminal integral membrane polypeptide.
(A and B) SAG1-C65-HA localizes to the cell body plasma membrane, with little on flagella and was not detectable in control hap2 gametes. (C) SAG1-C65-HA is expressed primarily as a 65 kDa C-terminal fragment and present in cell bodies with little in flagella. (D) Both flagellar and cell body SAG1-C65-HA are insoluble after freeze-thaw or incubation in 0.5 M NaCl, but are soluble in the non-ionic detergent, NP-40 (1%). (E) SAG1-C65-HA is degraded upon treatment of live gametes with 0.01% trypsin for 5 min (upper panel). The lower panel shows anti-tubulin staining.
The predominant form of SAG1-HA detected in cells is a 65 kDa C-terminal integral membrane polypeptide fragment, SAG1-C65-HA
Immunoblotting analysis showed that SAG1-HA gametes contained low amounts of a high molecular mass form of SAG1-HA, which we propose is newly synthesized, nearly full-length SAG1, but an HA-tagged 65 kDa C-terminal polypeptide fragment, SAG1-C65-HA, predominated. SAG1-HA was detectable only in gametes (Supplemental Figures S2 A–B). Our model is that full-length SAG1-HA is cleaved soon after synthesis to yield the aqueous-soluble SAG1 agglutinin and the C-terminal SAG1-C65-HA (Supplemental Figure S2C). Cell fractionation studies showed that SAG1-C65-HA was primarily a cell body protein, with variable, but typically low amounts in flagella (Figure 1C), confirming the immunofluorescence results that it was mostly excluded from the organelles in resting gametes.
Biochemical analysis (Figure 2D) confirmed transmembrane domain predictions (Supplemental Figure S2C) and showed that SAG1-C65-HA had the properties of an integral membrane protein. Both cell body and flagellar SAG1-C65-HA remained sedimentable after treatment with high salt or freezing and thawing, but were soluble in the detergent NP-40 (Figure 2D). SAG1-C65-HA was degraded on live gametes that were treated briefly with low concentrations of trypsin (Figure 2E), confirming the immunolocalization results that it was on the cell surface. Thus, SAG1-C65-HA was a tagged, integral membrane polypeptide that we could use to test for signaling-induced protein redistribution.
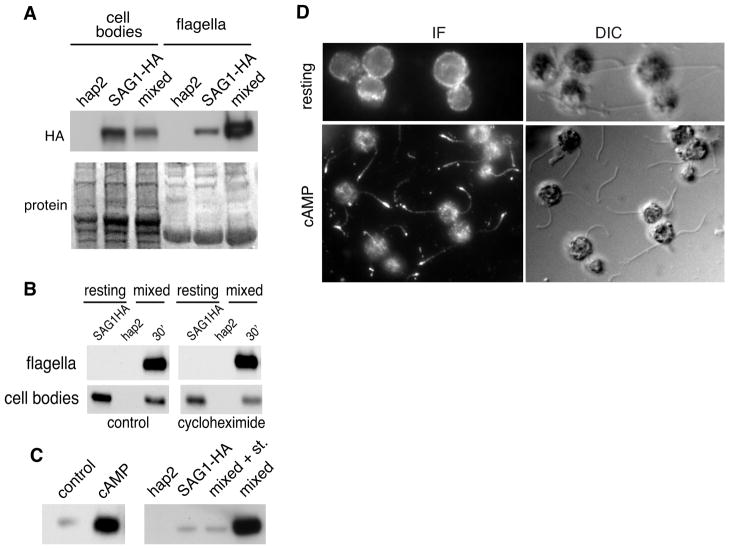
Figure 2. During gamete activation, pre-existing SAG1-C65-HA rapidly moves from the cell body to the flagella.
(A) SAG1-C65-HA becomes enriched in flagella during flagellar adhesion. The upper panel is an HA immunoblot and the lower panel shows the blot after staining for protein (9 μg protein/lane). (B) The SAG1-C65-HA that moves to flagella is derived from pre-existing SAG1-C65-HA. Immunoblots of cell bodies (7 × 105 cell equivalents [~7 μg protein] in each lane) and flagella (2 × 107 cell equivalents, [~3 μg protein] in each lane) isolated from SAG1-HA gametes mixed with hap2 minus gametes in the presence and absence of 10 μg/mL cycloheximide for the indicated times. Cells were pretreated for 30 min with CH before mixing. (C) Signaling is necessary and sufficient for rapid enrichment of SAG1-C65-HA in flagella. SAG1-C65-HA becomes enriched in flagella upon incubation of SAG1-HA gametes with db-cAMP (left panel; flagella from equal numbers of cells loaded in each lane). SAG1-HA gametes mixed with minus gametes in the presence of 1 μM staurosporine fail to enrich SAG1-C65-HA in their flagella (right panel; flagella from equal numbers of cells loaded in each lane). (D) Immunolocalization experiments show that SAG1-C65-HA becomes enriched in flagella of SAG1-HA gametes activated by incubation in db-cAMP (30 min).
Cell fractionation shows that flagellar adhesion-induced signaling triggers entry of SAG1-C65-HA into flagella
To determine whether SAG1-C65-HA moved to flagella during cilium-generated signaling, we mixed SAG1-HA plus gametes with fusion-defective hap2 minus gametes [31] for 10 min to activate the flagellar adhesion-induced signaling pathway and then isolated their flagella and cell bodies for immunoblotting. As shown in Figure 2A, almost immediately after signaling was induced by flagellar adhesion, the amount of SAG1-C65-HA in flagella rapidly and strikingly increased, with a concomitant reduction in cell body SAG1-C65-HA. To test whether protein synthesis was required for the signaling-induced increase in SAG1-C65-HA in flagella, we pre-incubated SAG1-HA plus gametes and hap2 minus gametes separately in the protein synthesis inhibitor cycloheximide (CH) for 30 min and then mixed them together in the continued presence of CH. Figure 2B shows that whereas SAG1-C65-HA was at very low levels (undetectable in these exposures) in the flagella isolated from cells before mixing, it was dramatically enriched in flagella after mixing. Moreover, the amount of SAG1-C65-HA in flagella was indistinguishable in control and cycloheximide-treated samples. Thus, reminiscent of earlier bioassay results on agglutinin [25, 28, 29], the SAG1-C65-HA that accumulated in flagella during signaling pre-existed on the cells.
Confirming the dramatic flagellar increase in SAG1-C65-HA triggered by adhesion-induced signaling, incubation of SAG1-HA plus gametes in the cell-permeable analogue of cAMP, dibutyryl cAMP (db-cAMP), which mimics the adhesion-induced signal, alone was sufficient to trigger entry of SAG1-C65-HA into flagella (Figure 2C, left panel). Accumulation was completely abrogated in cells undergoing flagellar adhesion in the presence of the protein kinase inhibitor, staurosporine (Figure 2C, right panel), which blocks signaling [23]. Immunolocalization studies confirmed the biochemical results showing regulated increase of SAG1-C65-HA in flagella. Strong HA staining became localized along the shafts of the flagella and at the flagellar tips in the db-cAMP-treated gametes (Figure 2D). Taken together, these results indicated that flagellar adhesion-induced signaling triggered redistribution of pre-existing SAG1-C65-HA from the plasma membrane onto the flagellar membrane.
The signaling-induced increase of SAG1-C65-HA in flagella does not require IFT
We took advantage of the Chlamydomonas temperature-sensitive kinesin 2 mutant fla10 (which has a point mutation in the motor domain) to test whether IFT was required for signaling-induced flagellar accumulation of SAG1-C65-HA. At the permissive temperature, fla10 cells [32, 33] possess flagella that are fully motile and functional. Upon shifting to the non-permissive temperature, the mutant FLA10 protein becomes non-functional and IFT particles no longer enter flagella. The flagella become depleted of the IFT machinery [23] within 45 min, but retain flagella of full length for another 30–45 min. We generated a SAG1-HA/fla10 cell line by crossing SAG1-HA gametes with fla10 minus gametes.
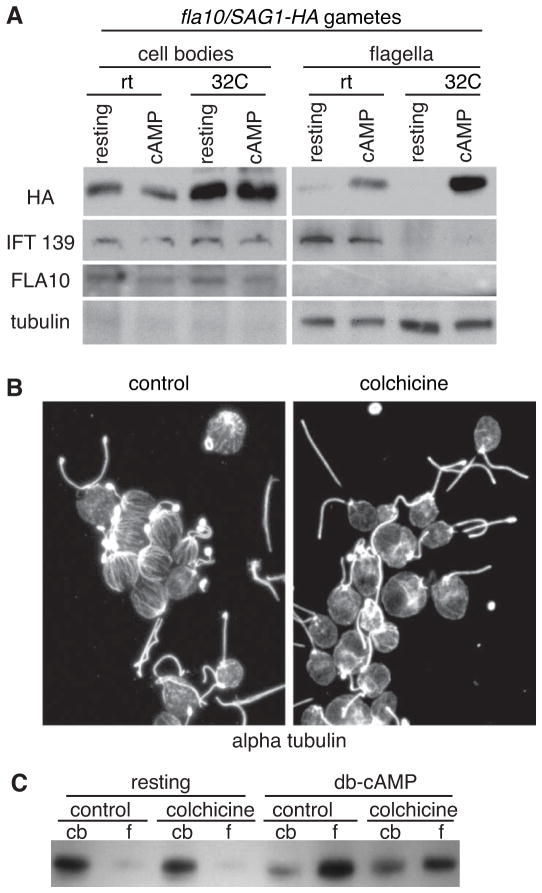
SAG1-HA/fla10 gametes were incubated at the non-permissive temperature for 45 min, incubated with db-cAMP to induce signaling [23], and cell bodies and flagella were analyzed by immunoblotting. As reported previously [32], even at the permissive temperature, fla10 cells possessed very low levels of the mutant FLA10 protein) in their flagella (Figure 3A). And, as expected, IFT complexes as measured by the IFT139 protein were depleted from the flagella of cells at the non-permissive temperature (32 °C). The equivalently low SAG1-C65-HA amounts in flagella of resting cells at the permissive and non-permissive temperatures indicated that IFT was not required for exclusion of the protein from flagella (Figure 3A). More importantly, even though their kinesin 2 had been rendered nonfunctional and their flagella were depleted of the IFT machinery at the non-permissive temperature, the SAG1-HA/fla10 gametes retained full ability to undergo signaling-induced flagellar enrichment of SAG1-C65-HA (Figure 3A). Therefore, regulated entry of SAG1-C65-HA into flagella did not depend on IFT.
Figure 3. Signaling-induced flagellar enrichment of SAG1-C65-HA is independent of IFT and facilitated by cytoplasmic microtubules.
(A) Immunoblots with the indicated antibodies of cell bodies and flagella (5 μg protein/lane) of SAG1-HA/ fla10 resting and activated gametes that had been incubated at the permissive and non-permissive temperatures for 45 minutes and then activated with db-cAMP for 5 min. (B) Incubation of cells for 100 min in 2 mg/ml colchicine disrupts the extensive array of cytoplasmic microtubules (right panel, control cells; left panel, colchicine treated cells; images are 2D projections of Z-stacks of confocal images of samples stained with anti-tubulin antibody). (C). Depletion of cytoplasmic microtubules impairs db-cAMP-induced flagellar enrichment of SAG1-C65-HA (10 min treatment with db-cAMP; 2 μg protein/lane).
Cytoplasmic microtubules facilitate the regulated increase of SAG1-C65-HA in flagella
The results above showing that SAG1-C65-HA became depleted from the cell bodies during adhesion (Figure 2A–B) raised the possibility that enrichment in flagella upon signaling was not simply due to passive equilibration between the plasma membrane and the flagellar membrane compartments, but was facilitated by signaling. With the exception of the actin filaments in the microvillus-like mating structure erected during gamete activation [22], these cytoskeleton elements are not detectable in Chlamydomonas vegetative cells or gametes and thus are unlikely to participate in flagellar enrichment. On the other hand, Chlamydomonas cells possess 15–20 microtubules that originate near the basal bodies and extend radially towards the posterior end of the cell, and that are intimately associated with the plasma membrane [34, 35] (Figure 3B, left panel; [see Supplemental Figure S2 from 35]). To test for a possible function of these microtubules in flagellar exclusion or enrichment of SAG1-C65-HA, we incubated cells with colchicine to disrupt the microtubules and examined SAG1-C65-HA distribution before and after incubation of the gametes in db-cAMP in the continued presence of colchicine. As expected [34], colchicine treatment did not alter the stable doublet microtubules of the flagella, but disrupted the singlet, cytoplasmic microtubules (Figure 3B, right panel). Immunoblot analysis showed that the amounts of SAG1-C65-HA in cell bodies and flagella from resting control cells were similar to those from resting, microtubule-depleted gametes, indicating that cytoplasmic microtubules were not required to exclude SAG1-C65-HA from flagella in resting cells (Figure 3C). On the other hand, although depletion of cytoplasmic microtubules did not completely block entry, db-cAMP-induced redistribution of SAG1-C65-HA from the cell body to the flagella was impaired (Figure 3C). Incubation of gametes with colchicine in the cold to bring about more complete depletion of microtubules led to an even greater inhibition of signaling-induced redistribution of SAG1-C65-HA to flagella (Supplemental Figure S4). These results demonstrated that cytoplasmic microtubules facilitated signaling-induced SAG1-C65-HA enrichment in flagella, indicating that global cellular events external to flagella participated in regulating flagellar membrane protein composition.
SAG1-C65-HA undergoes rapid, cytoplasmic microtubule-dependent redistribution to the apical plasma membrane during signaling
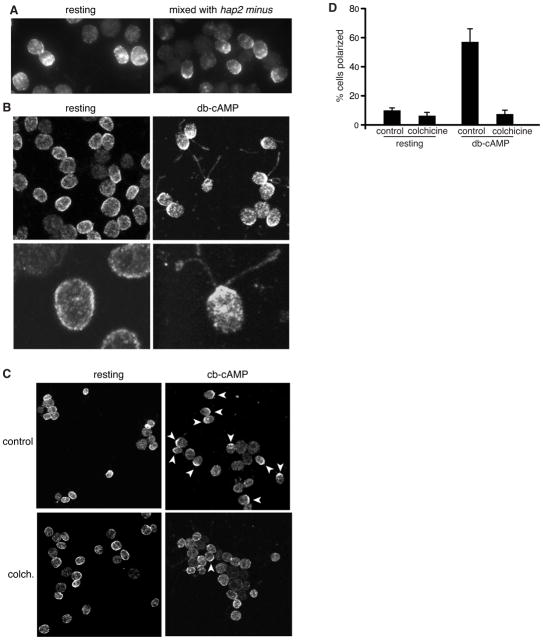
During immunofluorescence experiments with adhering SAG1-HA gametes we noticed that, not only did SAG1-C65-HA become enriched in the flagella, but in many cells it also became concentrated in the apical plasma membrane at the bases of the flagella (Figure 4A). The redistribution of SAG1-C65-HA to the peri-flagellar plasma membrane was even more striking in SAG1-HA gametes activated with db-cAMP. In most of the cells incubated for 5 minutes with db-cAMP, SAG1-C65-HA was depleted from the basal one half to two thirds of the cell and was highly enriched in the apical plasma membrane adjacent to the flagella as it became enriched in the flagella (Figure 4B; the lower panel shows a higher magnification view of activated gametes; see also Supplemental Movie S2).
Figure 4. Gamete activation triggers SAG1-C65-HA redistribution to the apical ends of the cells near the site of entry into flagella and polarization depends on cytoplasmic microtubules.
(A and B) Signaling induced by flagellar adhesion (A) (20 min mixing with hap2 minus gametes) or by 10 min incubation with db-cAMP (B) is accompanied by enrichment of SAG1-C65-HA at the apical plasma membrane. The lower panels in B are higher magnification views. (C and D) Apical enrichment is inhibited when cytoplasmic microtubules are disrupted by pre-incubation in colchicine. Upper left and right panels in (C) are control resting and db-cAMP-treated cells (respectively). Lower left and right panels in (C) are colchicine-treated resting and db-cAMP-treated cells (respectively). For the quantification shown in (D), a cell was scored as having apical accumulation of SAG1-C65-HA if the protein was depleted from the ~ lower 2/3 of the cell body plasma membrane and was concentrated at the apical end of the cell. Data are from 3 experiments; 100 cells were scored for each condition. Error bars indicate +/− SEM.
Having established that signaling triggered global redistribution of SAG1-C65-HA to the flagellar membrane entry site and that cytoplasmic microtubules facilitated SAG1-C65-HA entry into flagella, we tested whether cytoplasmic microtubules participated in polarization of SAG1-C65-HA. We incubated SAG1-HA gametes in colchicine and then in the continued presence of colchicine incubated them in db-cAMP. As shown in Figures 4C and 4D, SAG1-C65-HA redistribution to the peri-flagellar plasma membrane was inhibited in gametes depleted of cytoplasmic microtubules. Thus, cells use cytoplasmic microtubules during cilium-based signaling to concentrate SAG1-C65-HA at peri-flagellar sites as a part of its IFT-independent entry into the flagellar membrane.
DISCUSSION
Our studies provide combined immunofluorescence and biochemical evidence for large-scale, regulated trafficking to flagella of an integral membrane protein, SAG1-C65-HA. Cytoplasmic microtubules supported SAG1-C65-HA apical enrichment and flagellar entry. Importantly, SAG1-C65-HA redistribution to flagella did not depend on IFT, indicating that cells possess an as yet uncharacterized, regulated mechanism for passage of membrane proteins through the ciliary diffusion barrier. The even distribution of SAG1-C65-HA over the entire plasma membrane, its surface localization as assessed by trypsin sensitivity, and its relative paucity in the flagellar membrane suggested that the protein did not move freely between the two compartments in resting gametes. It will be interesting to determine whether Chlamydomonas BBSome proteins [36] or the many ciliary diffusion barrier proteins identified in Chlamydomonas and in other systems participate in regulating flagellar amounts of SAG1-C65-HA in resting gametes. Moreover, we cannot exclude the possibility that small amounts of SAG1-C65-HA continuously traffic through flagella. In the Hh pathway in vertebrates, Smo constitutively traffics through the ciliary membrane by a Patched-regulated mechanism that depends on retrograde IFT [19, 21] and treatments that increase cAMP also increase ciliary Smo [37]. Raising the possibility of additional similarities between cilium-based signaling in Chlamydomonas and vertebrates, recent studies indicate that transcripts for a Chlamydomonas Patched family member (Cre02.g093500) are many-fold higher in gametes compared to vegetative cells and are down-regulated during signaling [see Table S1 in 38].
Regulated enrichment of SAG1-C65-HA in flagella is independent of IFT
Our results that the increase in SAG1-C65-HA in flagella was independent of IFT (Figure 4A) were similar to an earlier report that restoration of flagellar adhesion in cells whose flagella had been experimentally rendered non-adhesive occurred in the absence of IFT [23]. The IFT-independent flagellar entry of SAG1-C65-HA we show here directly demonstrates that integral membrane proteins can pass through the ciliary diffusion barrier without IFT. It will be interesting to determine whether regulated ciliary accumulation of integral membrane proteins in other systems also might be independent of IFT.
Cytoplasmic microtubules participate in SAG1-C65-HA redistribution to the apical membrane and flagella
The model for entry that we favor is that adhesion-induced signaling brings about changes in the diffusion barrier or SAG1-C65-HA or both that allow IFT-independent SAG1-C65-HA passage through the barrier. The signaling-triggered ability to enter the flagellar membrane and the signaling-induced locally high concentration of SAG1-C65-HA at the site of entry into the flagella, then, could combine to support the accumulation in flagella.
In future studies it will be interesting to determine whether the relocalization of plasma membrane SAG1-C65-HA to the site of entry into the flagella derives from signaling-induced lateral movement or coupled internalization, vesicle trafficking, and exocytosis. To date there is little if any direct evidence for endocytosis in Chlamydomonas, which takes up only light and small molecule nutrients from its environment. In the Hh pathway, initial signaling-induced ciliary accumulation of Smo results from lateral movement of Smo pre-existing on the plasma membrane [37, 39]. Ciliary accumulation of later-appearing Smo from internal sources is not sensitive to disruption of microtubules by vinblastine [20], but whether the initial, lateral movement of Smo depends on microtubules has not been tested. Independently of the path SAG1-C65-HA follows during relocalization, it will also be interesting to determine whether cells use the retrograde IFT motor, cytoplasmic dynein 1b, for apical relocalization or if minus-end directed kinesins are required.
Our findings call into question the currently favored model that anterograde IFT is the sole mechanism for passage of membrane proteins through the ciliary/flagellar diffusion barrier at the base of the organelle and indicate that the barrier can be breached by other mechanisms. One possibility is that signaling-triggered changes in the properties of a membrane protein can endow it with a “passport” that allows it to negotiate passage through the diffusion barrier without the need for a motor.
EXPERIMENTAL PROCEDURES
Cells, cell culture, and reagents
Chlamydomonas reinhardtii strains 21gr (mt+; CC-1690), 6145C (mt−; CC-1961), sag1-5 [imp9] (mt+; CC-1146), fla10-1 and hap2 are available from the Chlamydomonas Resource Center. Vegetative growth of cells, production of gametes and zygotes, determination of cell wall loss, activation of gametes with db-cAMP, and experiments with the conditional fla10 mutant strain were as described previously. Use of the fusion-defective hap2 gametes, which undergo normal flagellar adhesion and adhesion-induced signaling but fail to fuse [31], allowed us to study the effects of signaling without interference from cell fusion. Unless otherwise noted, all chemicals were reagent grade and obtained from major suppliers.
Cloning and transformation
Transformation of sag1-5 flagellar adhesion mutants with a genomic form of the SAG1 gene is described in Supplemental Experimental Procedures. For some experiments, we also used SAG1-HA plus progeny from a cross between the original SAG1-HA cells and sag1-5 minus gametes. We generated a SAG1-HA/fla10/mt+ cell line by crossing SAG1-HA plus gametes and fla10 minus gametes and screening meiotic progeny for plus cells that expressed SAG1-C65-HA and shortened their flagella after several hours at 33 °C.
Cell fractionation
Cell bodies and flagella were isolated as previously described [24], resuspended to a concentration of ~2–4 mg/mL protein in 20 mM HEPES, 5 mM MgCl2, 1 mM DTT, 1 mM EDTA, 25 mM KCl; pH 7.2 or 10 mM Tris, ph 7.8, flash frozen in liquid nitrogen, and stored at −80 °C.
SDS-PAGE and immunoblotting
SDS-PAGE and immunoblotting were performed as previously described [24] using commercially available 4–20% polyacrylamide gels following the manufacturer’s protocol (GenScript USA). Sources and dilutions of antibodies are described in Supplemental Experimental Procedures.
Immunofluorescence microscopy
The immunofluorescence experiments were performed by attaching live cells to poly-L-lysine coated slides followed by fixation in methanol and processing as described in Supplemental Experimental Procedures. Samples were examined in a digital light microscope (Carl Zeiss Axioplan 2E, motorized focus drive with Hamamatsu monochrome digital camera) and a confocal microscope (Leica TCS SP5). OpenLab software was used to capture images. ImageJ software was used to analyze and process images.
Supplementary Material
01
02
03
Highlights.
- Chlamydomonas membrane polypeptide SAG1-C65 is on the cell body plasma membrane
- Flagellar signaling induces SAG1-C65 apical polarization and flagellar localization
- Cytoplasmic microtubules facilitate SAG1-C65 redistribution
- Movement of SAG1-C65 from the plasma membrane to flagella does not require IFT
Acknowledgments
We thank Neal Jetton for helping to generate the SAG1-HA/sag1-5 cells. We are indebted to Khrishen Cunnusamy (UT Southwestern) for his generosity in teaching us electroporation methods for transformation and to Amita Sahu (UT Southwestern) for assistance in several portions of this work. We thank Dennis Diener and Joel Rosenbaum (Yale University) for antibodies. This work was supported by NIH Grant GM-25661 to W. J. S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. New Engl J Med. 2011;364:1533–1543. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drummond IA. Cilia functions in development. Curr Opin Cell Biol. 2012;24:24–30. doi: 10.1016/j.ceb.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, et al. Mapping the NPHP-JBTS-MKS protein network reveals ciliopathy disease genes and pathways. Cell. 2011;145:513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol. 2012;14:61–72. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- 6.Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, et al. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet. 2011;43:776–784. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emmer BT, Maric D, Engman DM. Molecular mechanisms of protein and lipid targeting to ciliary membranes. J Cell Sci. 2010;123:529–536. doi: 10.1242/jcs.062968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachury MV, Seeley ES, Jin H. Trafficking to the ciliary membrane: how to get across the periciliary diffusion barrier? Annu Rev Cell Dev Biol. 2010;26:59–87. doi: 10.1146/annurev.cellbio.042308.113337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scholey JM. Intraflagellar transport motors in cilia: moving along the cell’s antenna. J Cell Biol. 2008;180:23–29. doi: 10.1083/jcb.200709133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hao L, Thein M, Brust-Mascher I, Civelekoglu-Scholey G, Lu Y, Acar S, Prevo B, Shaham S, Scholey JM. Intraflagellar transport delivers tubulin isotypes to sensory cilium middle and distal segments. Nat Cell Biol. 2011;13:790–798. doi: 10.1038/ncb2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohatgi R, Snell WJ. The ciliary membrane. Curr Opin Cell Biol. 2010;22:541–546. doi: 10.1016/j.ceb.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang K, Diener DR, Mitchell A, Pazour GJ, Witman GB, Rosenbaum JL. Function and dynamics of PKD2 in Chlamydomonas reinhardtii flagella. J Cell Biol. 2007;179:501–514. doi: 10.1083/jcb.200704069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin H, Burnette DT, Bae YK, Forscher P, Barr MM, Rosenbaum JL. Intraflagellar transport is required for the vectorial movement of TRPV channels in the ciliary membrane. Curr Biol. 2005;15:1695–1699. doi: 10.1016/j.cub.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 15.Trivedi D, Colin E, Louie CM, Williams DS. Live-cell imaging evidence for the ciliary transport of rod photoreceptor opsin by heterotrimeric kinesin-2. J Neurosci. 2012;32:10587–10593. doi: 10.1523/JNEUROSCI.0015-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ. IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev Cell. 2012;22:940–951. doi: 10.1016/j.devcel.2012.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell. 2006;17:3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev. 2010;24:2180–2193. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ocbina PJ, Eggenschwiler JT, Moskowitz I, Anderson KV. Complex interactions between genes controlling trafficking in primary cilia. Nat Genet. 2011;43:547–553. doi: 10.1038/ng.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim J, Kato M, Beachy PA. Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:21666–21671. doi: 10.1073/pnas.0912180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rohatgi R, Milenkovic L, Scott MP. Patched1 regulates hedgehog signaling at the primary cilium. Science. 2007;317:372–376. doi: 10.1126/science.1139740. [DOI] [PubMed] [Google Scholar]
- 22.Snell WJ, Goodenough UW. Flagellar adhesion, flagellar-generated signaling, and gamete fusion during mating. In: Witman GB, editor. The Chlamydomonas Sourcebook. 2. Vol. 3. New York: Elsevier; 2009. pp. 369–394. [Google Scholar]
- 23.Pan J, Snell WJ. Kinesin-II is required for flagellar sensory transduction during fertilization in Chlamydomonas. Mol Biol Cell. 2002;13:1417–1426. doi: 10.1091/mbc.01-11-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q, Pan J, Snell WJ. Intraflagellar transport particles participate directly in cilium-generated signaling in Chlamydomonas. Cell. 2006;125:549–562. doi: 10.1016/j.cell.2006.02.044. [DOI] [PubMed] [Google Scholar]
- 25.Snell WJ, Moore WS. Aggregation-dependent turnover of flagellar adhesion molecules in Chlamydomonas gametes. J Cell Biol. 1980;84:203–210. doi: 10.1083/jcb.84.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saito T, Tsubo Y, Matsuda Y. Synthesis and turnover of cell body agglutinin as a pool of flagellar surface agglutinin in Chlamydomonas reinhardtii gamete. Arch Microbiol. 1985;142:207–210. [Google Scholar]
- 27.Musgrave A, de Wildt P, van Etten I, Pijst H, Scholma C, Kooijman R, Homan W, van den Ende H. Evidence for a functional membrane barrier in the transition zone between the flagellum and cell body of Chlamydomonas eugametos gametes. Planta. 1986;167:544–553. doi: 10.1007/BF00391231. [DOI] [PubMed] [Google Scholar]
- 28.Hunnicutt GR, Kosfiszer MG, Snell WJ. Cell body and flagellar agglutinins in Chlamydomonas reinhardtii: the cell body plasma membrane is a reservoir for agglutinins whose migration to the flagella is regulated by a functional barrier. J Cell Biol. 1990;111:1605–1616. doi: 10.1083/jcb.111.4.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodenough UW. Cyclic AMP enhances the sexual agglutinability of Chlamydomonas flagella. J Cell Biol. 1989;109:247–252. doi: 10.1083/jcb.109.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferris PJ, Waffenschmidt S, Umen JG, Lin H, Lee JH, Ishida K, Kubo T, Lau J, Goodenough UW. Plus and minus sexual agglutinins from Chlamydomonas reinhardtii. Plant Cell. 2005;17:597–615. doi: 10.1105/tpc.104.028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Tewari R, Ning J, Blagborough AM, Garbom S, Pei J, Grishin NV, Steele RE, Sinden RE, Snell WJ, et al. The conserved plant sterility gene HAP2 functions after attachment of fusogenic membranes in Chlamydomonas and Plasmodium gametes. Genes Dev. 2008;22:1051–1068. doi: 10.1101/gad.1656508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lux FG, 3rd, Dutcher SK. Genetic interactions at the FLA10 locus: suppressors and synthetic phenotypes that affect the cell cycle and flagellar function in Chlamydomonas reinhardtii. Genetics. 1991;128:549–561. doi: 10.1093/genetics/128.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.LeDizet M, Piperno G. Cytoplasmic microtubules containing acetylated alpha-tubulin in Chlamydomonas reinhardtii: spatial arrangement and properties. J Cell Biol. 1986;103:13–22. doi: 10.1083/jcb.103.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ringo DL. Flagellar motion and fine structure of the flagellar apparatus in Chlamydomonas. J Cell Biol. 1967;33:543–571. doi: 10.1083/jcb.33.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol. 2009;187:1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. J Cell Biol. 2009;187:365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ning J, Otto TD, Pfander C, Schwach F, Brochet M, Bushell E, Goulding D, Sanders M, Lefebvre PA, Pei J, et al. Comparative genomics in Chlamydomonas and Plasmodium identifies an ancient nuclear envelope protein family essential for sexual reproduction in protists, fungi, plants, and vertebrates. Genes Dev. 2013;27:1198–1215. doi: 10.1101/gad.212746.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Zhou Z, Walsh CT, McMahon AP. Selective translocation of intracellular Smoothened to the primary cilium in response to Hedgehog pathway modulation. Proc Natl Acad Sci (USA) 2009;106:2623–2628. doi: 10.1073/pnas.0812110106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
01
02
03