Strategies, models and biomarkers in experimental non-alcoholic fatty liver disease research (original) (raw)
. Author manuscript; available in PMC: 2016 Jan 1.
Abstract
Non-alcoholic fatty liver disease encompasses a spectrum of liver diseases, including simple steatosis, steatohepatitis, liver fibrosis and cirrhosis and hepatocellular carcinoma. Non-alcoholic fatty liver disease is currently the most dominant chronic liver disease in Western countries due to the fact that hepatic steatosis is associated with insulin resistance, type 2 diabetes mellitus, obesity, metabolic syndrome and drug-induced injury. A variety of chemicals, mainly drugs, and diets is known to cause hepatic steatosis in humans and rodents. Experimental non-alcoholic fatty liver disease models rely on the application of a diet or the administration of drugs to laboratory animals or the exposure of hepatic cell lines to these drugs. More recently, genetically modified rodents or zebrafish have been introduced as non-alcoholic fatty liver disease models. Considerable interest now lies in the discovery and development of novel non-invasive biomarkers of non-alcoholic fatty liver disease, with specific focus on hepatic steatosis. Experimental diagnostic biomarkers of non-alcoholic fatty liver disease, such as (epi)genetic parameters and ‘-omics’-based read-outs are still in their infancy, but show great promise. . In this paper, the array of tools and models for the study of liver steatosis is discussed. Furthermore, the current state-of-art regarding experimental biomarkers such as epigenetic, genetic, transcriptomic, proteomic and metabonomic biomarkers will be reviewed.
Keywords: Non-alcoholic fatty liver disease, steatosis, models, drugs, biomarkers
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) covers a spectrum of chronic liver diseases, ranging from hepatic steatosis or fatty liver to non-alcoholic steatohepatitis (NASH), liver fibrosis, liver cirrhosis and eventually hepatocellular carcinoma (HCC). It is estimated that 1 billion people currently suffer from any form of NAFLD [1]. Liver steatosis, marked as the first stage of NAFLD, is closely associated with obesity, type 2 diabetes mellitus, insulin resistance (IR) and drug-induced liver injury [2]. This is substantiated by the fact that NAFLD is the most dominant chronic liver disease with a prevalence of 20-30 % in Western countries, 30-50 % in patients with type 2 diabetes mellitus and 80-90 % in obese adults [3-6]. Steatosis is considered as a benign reversible event, while NASH has the potential to lead to cirrhosis and HCC. As much as 10-25 % of liver steatosis patients progress to NASH and 2-3 % of NASH patients develop liver cancer [3, 7]. Hence, liver steatosis is of major concern for worldwide health, especially since sedentary lifestyle, modern Western nutrition, genetic predispositions, a variety of pharmacological agents and hepatitis B and C infection have been identified as critical causes of NAFLD [2, 3, 7-12]. At present, 70.000 Europeans are fatally affected by chronic liver disease every year [13]. Furthermore, HCC incidence increased tremendously over the past 25 years [14] and was responsible for the death of 21.670 patients in the United States in 2013 [15]. The economic burden imposed by chronic liver disease and its causes is considerable. Indeed, in the United States, 160 to 300 billion Dollars are spent every year on obesity, which constitutes 10 % of overall health care costs [16]. So far, the only curative therapy for acute and chronic liver failure is liver transplantation, with a cost in Europe of about 100.000 Euro the first year and 10.000 Euro yearly thereafter [17].
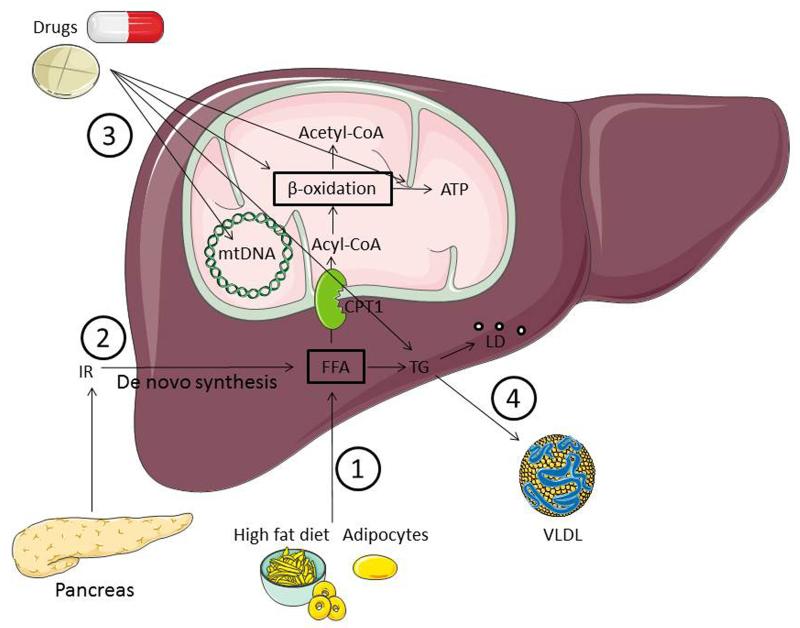
Liver steatosis is featured by the accumulation of fat in at least 5 % of hepatocytes. These fatty or lipid droplets, typically present in the cytosol, are composed of aggregated triglycerides (TGs) engulfed by a phospholipid monolayer [18]. In physiological conditions, these lipids generate energy and serve as a substrate for membrane synthesis, though in excess, this can lead to hepatocellular damage. Four pathogenic mechanisms are thought to be responsible for the accumulation of TG-based lipid droplets (Figure 1), namely (i) the increased uptake of free fatty acids (FFAs) from high-fat food or adipocytes in body fat, (ii) the increased synthesis of FFAs in the liver from glucose or acetate by IR, (iii) the decreased mitochondrial β-oxidation of FFAs caused by a multitude of drugs and (iv) the decreased secretion of TGs in very low density lipoproteins from the liver [11, 19]. The progression from steatosis to NASH is presented in the so-called “two-hit” hypothesis [20]. TG accumulation by IR, increased fatty acid influx, decreased fatty acid oxidation and/or decreased TG transport can be considered as the “first hit”, making the liver more sensitive to a “second hit”, such as oxidative stress, lipoapoptosis and increased production of inflammatory cytokines. This can ultimately result in the development of NASH [7, 20, 21]. Being associated with obesity and type 2 diabetes mellitus, IR plays a key role in the origin and maintenance of hepatic steatosis. Increased peripheral lipolysis and downregulation of tissue glucose uptake results in an increase of FFAs in hepatocytes and thus creates the basis for TG production. Together with decreased apolipoprotein synthesis, TG secretion, in the form of very low density lipoproteins, is inhibited. The resulting excess of FFAs induces a rise in mitochondrial β-oxidation, production of reactive oxygen species and oxidative stress as well as elevated lipid peroxidation, all which are hallmarks of the injury observed in the liver of obese patients suffering from steatosis [22-24]. Additionally, hyperinsulinemia and hyperglycemia, both caused by increased insulin secretion by pancreatic β-cells, trigger the transformation of FFAs to glucose and inhibit β-oxidation, resulting in the accumulation of TGs in the cytosol of hepatocytes. Collectively, these triggers induce the production of proinflammatory cytokines via release of tumor necrosis factor α, which leads to the development of NASH [11, 25-28]. The development of liver steatosis is also closely related to the metabolic syndrome. In this respect, liver steatosis patients show one or more features of the metabolic syndrome, such as IR, glucose intolerance, obesity, hypertriglyceridemia and elevated blood pressure [29]. Additionally, pharmacological agents targeting mitochondria are known to cause oxidative stress or liver steatosis (Figure 2).
Figure 1. Pathogenesis of steatosis.
Four pathogenic mechanisms can be responsible for the accumulation of TG-based lipid droplets, namely 1) increased uptake of FFAs from high-fat food or from adipocytes in body fat, 2) increased synthesis of FFAs in the liver from glucose or acetate by IR, 3) decreased mitochondrial β-oxidation of FFAs caused by a multitude of drugs, and 4) decreased synthesis or secretion of very low density lipoproteins, the principal route for elimination of lipids from the liver. (ATP, adenosine triphosphate; CoA, coenzyme A; FFA, free fatty acids; LDs, lipid droplets; IR, insulin resistance; TGs, triglyceride; VLDL, very low density lipoproteins).
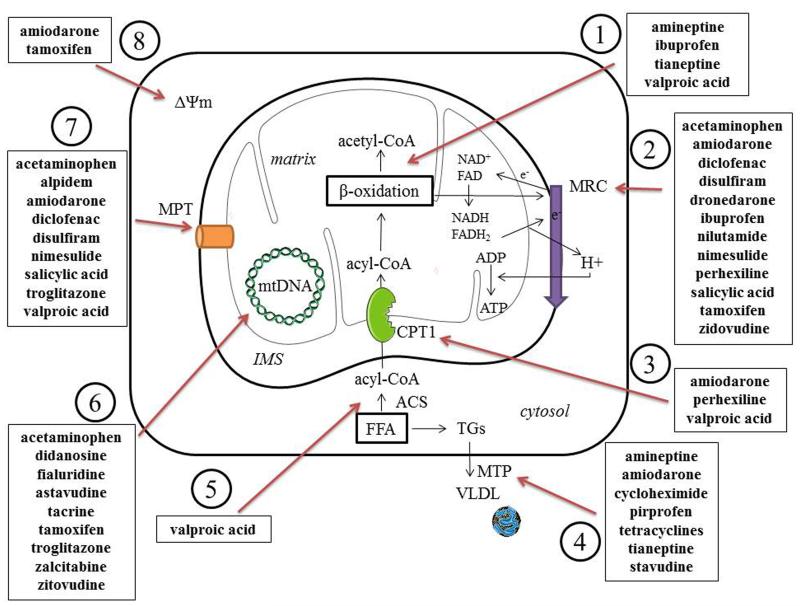
Figure 2. Mechanisms of drug-induced liver steatosis.
Drugs may induce liver steatosis by a plethora of mechanisms associated with mitochondrial impairment, including 1) inhibition of enzymes involved in mitochondrial FFA oxidation leading to a direct alteration in β-oxidation function, 2) direct inhibition of the mitochondrial respiratory chain reaction or alteration of oxidative phosphorylation, 3) decreased uptake of acyl-CoA due to the inhibition of carnitine palmitoyl transferase 1, 4) reduction in triglyceride excretion by inhibition of microsomal triglyceride transfer protein, 5) impaired entrance of fatty acids in mitochondria due to the inhibition of acyl-CoA synthase, 6) depletion of mitochondrial DNA, encoding for mitochondrial proteins, which leads to impairment of MRC, 7) induction of the mitochondrial permeability transition pore opening, 8) alteration of mitochondrial membrane potential. (ACS, acyl-coenzyme A synthase; ADP, adenosine diphosphate; ATP, adenosine triphosphate; CoA, coenzyme A; CPT1, carnitine palmitoyl transferase 1; FAD, oxidized flavin adenine dinucleotide; FADH2, reduced flavin adenine dinucleotide; FFA, free fatty acids; IMS, intermembrane space; MRC, mitochondrial respiratory chain; MPT, mitochondrial permeability transition; MTTP, mitochondrial triglyceride transfer protein; NAD, oxidized nicotinamide adenine dinucleotide; NADH, reduced nicotinamide adenine dinucleotide; TG, triglyceride; VLDL, very low density lipoproteins).
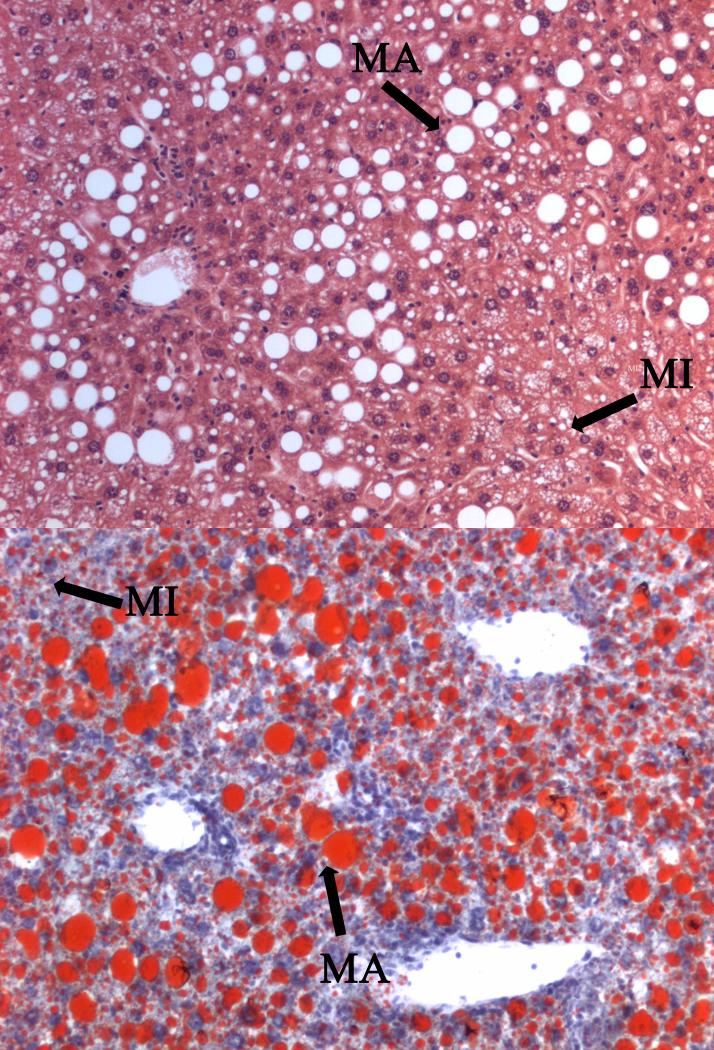
Histologically, no distinction can be made between alcoholic fatty liver disease and NASH, since both conditions have an identical histological pattern. This emphasizes the importance of excluding significant alcohol intake (i.e. more than 20 gram per day) in patients when diagnosing NASH. The latter is characterized by the presence of steatosis, mixed inflammatory cell infiltration, hepatocyte ballooning, necrosis, glycogen nuclei, Mallory’s bodies and fibrosis [30]. Two types of steatosis can be recognized, namely macrovesicular steatosis and microvesicular steatosis. Both present the accumulation of TGs in the cytosol of hepatocytes. However, in case of macrovesicular steatosis, large lipid droplets which displace the cytoplasmic content and nucleus are observed, while in microvesicular steatosis small well-circumscribed lipid droplets that do not displace the nucleus are seen (Figure 3) [31].
Figure 3. Histological patterns of liver steatosis.
Hematoxylin and eosin (upper panel) and Oil Red (lower panel) stained liver slice from C57BL/6 mice fed a high-fat choline-deficient diet for 8 weeks developing mixed macrovesicular and microvesicular steatosis. Both pictures depict the accumulation of TGs in the cytosol of hepatocytes. However, in case of macrovesicular steatosis, large lipid droplets are observed, which displace the cytoplasmic content and nucleus, while in microvesicular steatosis, small lipid droplets that do not displace the nucleus are seen (MA, macrovesicular steatosis; MI, microvesicular steatosis).
Steatosis is often accompanied by phospholipidosis, implying the intracellular accumulation of phospholipids. As such, phospholipidosis is a lysosomal storage disorder and is considered as a reversible side effect [32]. Phospholipidosis will not be further discussed in this paper. Rather, focus will be put on strategies to experimentally mimic the pathological features of NAFLD induced by drugs, diets or dietary ingredients combined with rodents, zebrafish and genetic modified animals as well as with liver-based in vitro models. The clinical diagnosis of liver steatosis and NAFLD is based on a number of read-outs, including clinical chemistry parameters (e.g. cholesterol and transaminase levels), histopathological examination of liver biopsies and specific physical tests (e.g. computed tomography and nuclear magnetic resonance) [33]. These clinical read-outs will not be considered in this paper. Instead, particular attention will be paid to more experimental diagnostic biomarkers of NAFLD, such as (epi)genetic parameters and ‘-omics’-based read-outs.
2. Strategies in NAFLD research
2.1. Drug treatment
2.1.1. Valproic acid
With its anticonvulsive activity being discovered in 1963, valproic acid (VPA), or its sodium valproate derivative, is currently the most commonly prescribed anti-epileptic drug worldwide. In addition to its anticonvulsant properties, VPA is also used for the treatment of bipolar disorders [34-36], clinical depression [37], absence seizures [38], tonic-clonic seizures [39], complex partial seizures [40] and juvenile myoclonic epilepsy [41]. More recently, VPA has been characterized as a histone deacetylase inhibitor [42] with therapeutic potential in the cancer field. Although VPA is considered as a drug with a relatively safe profile, adverse reactions, such as encephalopathy, hypersensitivity syndrome and teratogenicity, have been reported in patients receiving VPA [43]. One of the most alarming side effects is hepatotoxicity, in particular microvesicular steatosis, caused by impairment of mitochondrial β-oxidation. Indeed, upon sequestration with coenzyme A (CoA) to valproyl-CoA in the cytosol of hepatocytes, VPA can enter mitochondria. This results in the inhibition of mitochondrial fatty acid oxidation, since VPA undergoes oxidation to several products and hereby competes with endogenous lipids for enzymes in the oxidation pathway [44-46]. The development of microvesicular steatosis typically occurs in mice given doses of VPA ranging from 100 mg/kg/day for 2 weeks to 1000 mg/kg/day for 24 hours [47, 48]. The first step in the bioactivation pathway of VPA is the dehydrogenation by cytochrome P450 (CYP) 2C9 and CYP2A6 enzymes, forming 4-ene-valproate. This triggers an additional mechanism of β-oxidation inhibition. In vivo and in vitro studies have shown that 4-ene-valproate is a more steatogenic and cytotoxic compound compared with the parent drug [49-51]. Furthermore, 4-ene-valproate is transformed to 2,4-diene-valproyl-CoA, which may inactivate β-oxidation enzymes [52, 53]. VPA also acts as an anionic uncoupler by translocating protons in the mitochondrial matrix. This protonophoric effect can slightly uncouple mitochondrial respiration and may induce opening of the mitochondrial permeability transition pore, serving as another stimulus of VPA-induced cell death [54]. Recently, a new mechanism of VPA-induced hepatic steatosis has been described, namely inhibition of carnitine palmitoyl transferase 1, which transfers FFA into the mitochondria, by VPA-CoA. This results in decreased mitochondrial uptake of FFA and thus leads to a rise in TG accumulation [55].
2.1.2. Amiodarone
Amiodarone is a large amphiphilic drug used for the treatment of atrial and ventricular arrhythmias. Long-term therapy with amiodarone is limited by a multitude of extracardiac side effects, such as pulmonary toxicity [56], skin hyperpigmentation [57], thyroid dysfunction [58] and liver failure [59] due to the inhibition of mitochondrial β-oxidation [60-62]. Hepatic steatosis usually occurs in mice at a dose of 150 mg/kg/day when treated for 4 to 7 days [63]. In its unprotonated form, amiodarone can easily move across the outer mitochondrial membrane. Upon protonation, it diffuses through the inner mitochondrial membrane. Inside the relatively alkaline mitochondrial matrix, amiodarone loses its protonated status, which as such decreases the membrane potential [60, 64]. Furthermore, amiodarone equally inhibits microsomal triglyceride transfer protein [65], able to transfer TGs and assemble them into very low density lipoproteins and chylomicrons [66]. Dronedarone, an amiodarone-like anti-arrhythmic drug, was proposed as an alternative for amiodarone for the treatment of atrial fibrillation and was alleged to be less hepatotoxic. Nevertheless, it has been demonstrated that dronedarone has at least the same potential to inhibit the mitochondrial respiratory chain and β-oxidation [67].
2.1.3. Tetracyclines
The tetracyclines, a family of broad-spectrum antibiotics, are widely used in the therapy of human and animal infections due to their activity against gram-positive and gram-negative bacteria and atypical organisms such as chlamydiae and mycoplasmas [68]. Tetracyclines were among the first drugs found to induce microvesicular steatosis, typically becoming manifested 4 to 10 days after the first intravenous administration of high doses [69, 70]. In mice, microvesicular steatosis is caused by doses of 100 to 200 mg/kg/day [71, 72]. The main mechanism responsible for the development of hepatic steatosis relies on decreased evacuation of TGs by inhibition of mitochondrial triglyceride transfer protein, as tetracyclines are rapidly taken up by mitochondria in vitro and in vivo [70, 73, 74]. Different cases of fatal hepatotoxicity during tetracycline or minocycline therapy [75, 76] together with the development of oral alternatives and the emergence of resistance strains of bacteria in the last decades have led to a decrease in the use of intravenous tetracyclines [77].
2.1.4. Tamoxifen
Tamoxifen is the golden standard in the treatment of estrogen-related breast cancer in women. It is a selective estrogen receptor modulator and can act both agonistic and antagonistic, executing a multitude of beneficial and detrimental effects, including downregulated low density lipoprotein levels [78], an increase in bone mineral density [78], promoted cardioprotection [79] and a higher risk for the development of endometrial cancer [80]. Tamoxifen is an estrogen receptor antagonist in breast tissue and therefore can block estrogen signaling leading to a decrease in breast cancer death rate [81]. Another well-known side effect of tamoxifen includes the occurrence of liver steatosis [82-84]. This is found in mice treated with 150 mg/kg tamoxifen for 24 hours or for 2 weeks with 40 mg/kg/day tamoxifen [85, 86]. Like amiodarone, tamoxifen is an amphiphilic drug that is protonated in the intermembrane space and unprotonated in the matrix of mitochondria following transportation over the inner membrane. The subsequent release of protons uncouples oxidative phosphorylation. In fact, tamoxifen compromises the electron transport chain and decreases the regeneration of oxidized cofactors, such as nicotinamide adenine dinucleotide and flavin adenine dinucleotide [87, 88].
2.1.5. Non-steroidal anti-inflammatory drugs
The analgesic, antipyretic and anti-inflammatory drug acetylsalicylic acid is known to cause Reye’s syndrome [89], an often fatal complication in children characterized by vomiting, high fever, encephalopathy and hepatic failure [90]. The latter is accompanied by accumulation of lipid droplets in hepatocytes and is mostly due to salicylate, the main toxic metabolite of acetylsalicylic acid. Salicylate causes mitochondrial swelling, uncoupling of oxidative phosphorylation, impairment of mitochondrial respiration and inhibition of adenosine triphosphate (ATP) synthesis [91-93]. Other non-steroidal anti-inflammatory drugs, such as ibuprofen and pirprofen, were also found to interfere with mitochondrial β-oxidation and to produce microvesicular steatosis. Both enantiomers of ibuprofen inhibit β-oxidation and oxidative phosphorylation [94], but only the _R_-enantiomer can sequester CoA and may stereoselectively inhibit the uptake and oxidation of fatty acids [95]. By contrast, pirprofen suppresses mitochondrial triglyceride transfer protein activity [96]. Furthermore, both diclofenac and nimesulide have the potential to inhibit oxidative phosphorylation and to induce mitochondrial permeability transition pore opening [97]. Acetaminophen damages mitochondrial DNA, is able to open the mitochondrial permeability transition pore and inhibits oxidative phosphorlylation [98-100].
2.1.6. Miscellaneous drugs
Methotrexate is a drug used for the treatment of rheumatoid arthritis, psoriasis, psoriatic arthritis, inflammatory bowel disease and Crohn’s disease [101, 102]. It acts through the inhibition of folic acid production, a key regulator in the synthesis of RNA and DNA. Methotrexate can enter the cell via the organic anion-transporting polypeptide 1B1 [103] and inhibits the respiratory chain [104]. Patients displaying risk factors, such as obesity and diabetes, are more prone to the hepatotoxic characteristics of methotrexate and may develop steatohepatitis-like histological patterns [105]. Irinotecan is a topoisomerase 1 inhibitor and a very active antineoplastic drug. It is a component of modern chemotherapy regimens for lung and colorectal cancer treatment. Irinotecan use has been associated with the development of NASH [106]. Amineptine and tianeptine, both tricyclic antidepressant drugs, impair β-oxidation and cause microvesicular steatosis in mice in large doses [107, 108]. Besides the direct inhibition of FFA oxidation, both drugs also negatively affect mitochondrial triglyceride transfer protein activity [96]. The antiretroviral nucleoside reverse-transcriptase inhibitors stavudine and zidovudine constitute important drugs in the therapy of human immunodeficiency virus-infected patients. They can cause hepatic steatosis by depleting mitochondrial DNA [109], a mechanism equally reported for didanosine and zalcitabine [110]. Perhexiline is a prophylactic anti-anginal agent that inhibits oxidative phosphorylation [64] and carnitine palmitoyl transferase 1 [111]. Patients taking perhexiline maleate show mild to moderate fatty changes in the liver together with steatohepatitis [112]. Cycloheximide, which impedes the binding of tRNA to mRNA, induces liver steatosis in rats [113]. Several studies have demonstrated that this may be caused by enhanced hepatic FFA uptake, disturbed balance between FFA oxidation and esterification and inhibition of very low density lipoproteins secretion [114, 115]. Recently, cycloheximide has been shown to decrease hepatic lipid secretion due to acute apolipoprotein B reduction, a lipoprotein responsible for the packaging of very low density lipoproteins [116]. Other drugs altering mitochondrial functions (see Figure 2) include disulfiram (i.e. mitochondrial permeability transition pore opening and inhibition of mitochondrial respiratory chain reaction) [117-119], fialuridine (i.e. inhibition of mitochondrial DNA synthesis) [120], alpidem (i.e. mitochondrial permeability transition pore opening) [121], nilutamide (i.e. inhibition of mitochondrial respiratory chain reaction) [122], troglitazone (i.e. damaging of mitochondrial DNA and mitochondrial permeability transition pore opening) [123, 124], tacrine (i.e. impairment of oxidative phosphorylation and inhibition of mitochondrial DNA synthesis) [125, 126], buprenorphine (i.e. inhibition of oxidative phosphorylation and mitochondrial respiratory chain reaction) [127], orotic acid [128], clozapine [129] and thioacetamide [130].
2.2. Diet treatment
2.2.1. High-fat diet
A high-fat diet (HFD), consisting typically of 30 to 60 % fat, is commonly used to experimentally induce human-relevant NAFLD in rodents. However, the amount of fat, the composition of the HFD and the duration of the diet regime may cause different responses with respect to obesity, impaired glucose tolerance, dyslipidemia, increased lipogenesis, production of proinflammatory cytokines and oxidative stress depending on the species, strain and gender [131, 132]. In this respect, a diet containing high quantities of unsaturated fat leads to more pronounced hepatic lipid peroxidation as well as a higher expression of chemokines and cytokines compared to saturated fat-based diets [133]. Furthermore, a lard-rich HFD has much more impact on hepatic insulin sensitivity than a palm oil-rich HFD in mice [134]. The HFD most closely resembles the human pathology of NAFLD, as it is associated with IR, obesity and a similar histological pattern of liver steatosis.
2.2.2. Methionine-choline-deficient diet
Methionine and choline play a role in hepatic β-oxidation and the production and secretion of very low density lipoproteins [135]. They cannot be de novo synthesized and therefore must be included in the diet. A methionine-choline-deficient (MCD) diet consists of 40 % sucrose and only 10 % fat, and lacks methionine and choline. Feeding mice an MCD diet results in the onset of hepatic steatosis after 1 or 2 weeks due to increased uptake of fatty acids and altered secretion of very low density lipoproteins [135]. Subsequently, NASH is developed and is accompanied by oxidative stress [136], production of cytokines and adipocytokines [137], and fibrosis [136]. Although NAFLD is developed very rapidly, these mice lack IR and do not display overweight, which mirrors less the human pathology of NAFLD. In fact, several studies showed a severe weight loss in mice on MCD diet [138]. Although these animals abundantly present inflammation, oxidative stress and fibrosis, the response of the MCD diet may vary depending on species, strain and gender [139]. Compensating the lack of weight gain and IR can be done by feeding rodents a choline-deficient L-amino acid diet, which also causes NAFLD [140].
2.2.3. Lipogenic methyl-deficient diet
Mice fed a methyl-deficient diet for 6, 12 or 18 weeks develop liver injury similar to NASH, characterized by the presence of steatosis, inflammation and hepatocellular degeneration. These liver effects are progressive with duration of the diet, being most severe in the DBA/2J mice fed a methyl-deficient diet for 18 weeks. Moreover, the level of triglycerides in DBA/2J mice was greater than in C57BL/6J mice. Feeding mice a methyl-deficient diet resulted in a change in DNA methylation and profound alterations in histone modification patterns [141, 142].
2.2.4. Fructose-rich diet
Fructose is one of the most important sugars in human diet and is a major inducer of hepatic steatosis in rodents. It is taken up into the portal system and further by hepatocytes via glucose transporter members 2 and 5. Thereafter, fructose is metabolized in the glycolytic and lipogenic pathways. Fructose is known to harm the liver and to cause IR, oxidative stress, increased cytokine and adipocytokine levels and obesity [143, 144]. Thus, rats fed a 70 % fructose diet for 5 weeks produce macrovesicular steatosis, inflammation and high TG concentrations in the liver [145]. Likewise, after 16 weeks of high-fructose diet in mice, increased lipogenic proteins, increased serum transaminases, mixed macrovesicular and microvesicular steatosis as well as NASH are observed [146].
2.2.5. Fast food diet
A Western style diet or fast food diet is a combination of fat, cholesterol and sugar, which are acknowledged risk factors for the development of obesity. Hence, this diet can be used in rodents to induce NAFLD. When feeding mice a fast food diet consisting of 40 % fat and 2 % cholesterol combined with fructose-rich drinking water for 6 months, overweight, IR and NASH are produced [147]. Similarly, mice fed a liquid fast food diet for 6 weeks show massive hepatic steatosis and inflammation [132].
2.2.6. Dietary fatty acids
Oleic acid, a naturally occurring mono-unsaturated fatty acid, is one of the most common fatty acids in human diet [148-155]. It is indispensable for the formation of the plasma membrane and acts as an energy source. Fatty acids are metabolized in the mitochondria and serve as suppliers of ATP in the mitochondrial respiratory chain. Despite this critical physiological role, oleic acid is the most dominant fatty acid observed in liver steatosis patients [156]. The uptake of fatty acids, including oleic acid and palmitic acid, by hepatocytes takes place after dissociation from serum albumin. The main plasma membrane transporters that underlie this process are fatty acid-binding protein, cluster of differentiation 36 (CD36)/fatty acid translocase, fatty acid transport protein and caveolins [157-161]. After cytosolic fatty acid conjugation with acyl-CoA, the fatty acids can be transferred inside the mitochondria by means of carnitine palmitoyl transferase 1 [162, 163]. Under physiological conditions, intracellular fatty acid levels are low as a result of mitochondrial β-oxidation or esterification to triglycerides [164-166]. Oleic acid and palmitic acid are often used in in vitro to induce fat accumulation in cultured cells, with the former acting more potent than the latter [163, 167]. Common concentrations of oleic acid and palmitic acid used can range from 0.05 to 2 mM, depending on the duration of exposure [163, 168-170].
3. Models in NAFLD research
3.1. Animal models
3.1.1. Rodents
Rodents are currently the most routinely used in vivo models in liver steatosis research. Nevertheless, major differences exist in the development of mitochondrial injury, the progression of liver steatosis and the production of inflammatory markers and TGs between species, strains and genders. Thus, male rats fed an MCD diet or HFD produce more steatosis, and have a higher liver lipid content and serum alanine aminotransferase quantities than female rats. Male and female Wistar rats show higher liver lipid amounts, serum alanine aminotransferase levels and liver mass-body mass ratios compared to Long-Evans and Sprague Dawley counterparts. Male C57/BL6 mice show more inflammatory foci, products of lipid peroxidation and mitochondrial injury, but less steatosis and lower hepatic TG levels than Wistar rats [171, 172]. Unlike Sprague Dawley rats that exhibit macrovesicular steatosis accompanied by fibrosis, Lewis rats present microvesicular steatosis after receiving a HFD for 3 weeks. Also, lower cholesterol, TGs and leptin levels are measured in female Lewis rats compared to male rats of the same strain [172].
The induction of steatosis by VPA associated with microvesicular fat deposition in the liver was first shown in male Crl Swiss mice [173]. Sprague Dawley rats repeatedly exposed to VPA produce liver steatosis correlating with lipid accumulation, preferably in the perivenous zone [174]. In contrast, daily administration of VPA to male Sprague Dawley results in combined macrovesicular and microvesicular steatosis located in the periportal area [175]. ICR mice develop microvesicular steatosis after receiving tetracycline [71], and a time-dependent and dose-dependent increase in TGs is seen after treatment with VPA [47, 176]. Female Sprague Dawley rats daily fed tamoxifen display a rise in hepatic TG levels by more than 50 % after 14 days. However, activities and mRNA levels of enzymes involved in β-oxidation, ketogenesis and uptake of lipids from liver are unaffected, whereas the uptake of fatty acids is decreased [86]. Furthermore, female Wistar rats and jvs mice show extensive microvesicular and focal macrovesicular steatosis after repeated VPA administration [48, 177]. Male C57BL/6 mice have been used in a number of studies, whereby hepatic fat accumulation in the liver was observed following administration of tamoxifen [85] or amiodarone [63]. Swiss male mice treated with irinotecan manifest steatosis, lobular neutrophil infiltration and ballooning, portal neutrophil infiltration, interleukin-1β and fibrosis in liver tissue [178].
3.1.2. Zebrafish
In recent years, the zebrafish model for studying lipid metabolism has gained tremendous popularity because of the relatively low cost, ease of genetic manipulation, molecular homology with humans and rapid development of liver pathology. Zebrafish express adiponectin receptors [179] and the 3 peroxisome proliferator-activated receptor (PPAR) isoforms (α, β, γ) [180], all of which are essential to lipid homeostasis. Injection of thioacetamide in zebrafish 3 times a week for 2 weeks causes an increase of hepatic lipid content. However, serum TGs are not significantly increased, while tumor necrosis factor α and adiponectin receptor 2 mRNA expression is elevated and downregulated, respectively [130]. When adding thioacetamide to the rearing water of zebrafish embryos, necrotic fatty hepatocytes are observed. This is associated with strong transcriptional upregulation of adiponectin and mild to moderate upregulation of peroxisomal thiolase (i.e. β-oxidation related gene), CCAAT/enhancer binding protein α and β (i.e. transcription factors which play a role in adipogenesis) and sterol regulatory element-binding protein 1c (i.e. transcription factor that regulates lipid synthesis) [181]. Zebrafish larvae exposed to tunicamycin develop hepatomegaly and hepatic steatosis. In this model, hepatic stellate cells become activated, suggesting the onset of liver fibrosis [182]. Daily feeding of zebrafish with Artemia results in obesity characterized by an increase in body weight, plasma TGs and blood glucose after 2 weeks [183, 184]. Similarly, fructose-treated zebrafish larvae develop liver steatosis [185].
3.1.3. Genetically modified rodents and zebrafish
Leptin is a key regulator of lipid storage by adjusting the sensation of hunger. Leptin-deficient or ob/ob mice are hyperphagic, extremely obese, develop IR with hyperinsulinemia and hyperglycemia, and have a fatty liver [186]. However, they do only develop NASH upon a “second hit”, such as the administration of lipopolysaccharide [187] or feeding a HFD or MCD diet. A similar model is the db/db mouse, carrying a mutation in the leptin receptor gene. Db/db mice develop steatosis, are obese and diabetic and also need a “second hit” to develop NASH [188]. Attenuated P2 diphtheria toxin A-transgenic mice are hyperphagic, have low leptin levels, and show hyperlipidemia, hyperglycemia and IR [189]. Mice deficient in the melanocortin 4 receptor, a regulator of food intake and lipid metabolism, present NASH, but need a HFD to progress to liver fibrosis and HCC. Likewise, mice in which the expression of sterol regulatory-element binding protein 1a, a determinant of fatty acid synthesis and regulation of lipogenic genes, is overexpressed have an enlarged steatotic liver [190, 191], are hyperphagic because of low leptin levels and develop hepatic steatosis, IR and increased TG levels [192]. CD36-null mice exhibit increased plasma fatty acids and TG levels [193]. Recently, liver-specific overexpression of insulin-like growth factor 2 mRNA binding protein 2-2 p62 was shown to induce steatosis in mice receiving regular chow diet. Additionally, NASH-induced fibrosis was amplified in p62-transgenic mice fed a MCD diet.
In both models, hepatic cholesterol and monounsaturated fatty acids were increased in comparison with wild-type animals [194]. This is in line with other studies exhibiting the role of p62 in human HCC [195] and steatosis [196, 197]. A multitude of studies confirmed the association between a sequence polymorphism in patatin-like phospholipid domain-containing protein 3 (PNPLA3) on the 148M allele and the full spectrum of NAFLD [198]. After being fed a high-fructose diet, PNPLA3/148M-transgenic mice exhibit TG accumulation in the liver [199]. Furthermore, apolipoprotein E2 knock-in mice were found to be hyperlipidaemic. When fed a HFD, female apolipoprotein E2 knock-in mice show higher expression of lipogenic, cholesterol-synthesizing, inflammatory and cell-stress genes in comparison with female wild-type mice or male apolipoprotein E2 knock-in mice [200]. Several other genetically modified mice have been described, all which are pertinent to liver steatosis research. Their characteristics are summarized in Table 1.
Table 1. Genetically altered animal models of NAFLD.
AdipoR, adiponectin receptor; AOX, acyl-coenzyme A oxidase; AP2diph, Attenuated P2 diphtheria toxin A; ApoE, apolipoprotein E; CBS, Cystathionine-β-synthase; CBR, cannabinoid receptor; CD36, cluster of differentiation 36; CDIPT, CDP-diacylglycerol- inositol 3-phosphatidyltransferase; FXR, farnesoid x receptor; Gal, galectin; GMPS, guanosine monophosphate synthetase; HCC, hepatocellular carcinoma; HFD, high-fat diet; IL-6, interleukine-6; IR, insuline resistance; LDL(r) nicotinamide adenine dinucleotide (receptor), low density lipoprotein; MC4, melanocortin 4; MAT, methionine adenosyl transferase; MTP, mitochondrial trifunctional protein; NASH, non-alcoholic steatohepatitis; PPAR, peroxisome proliferator-activated receptor; PECAM, platelet endothelial cell adhesion molecule; PNPLA, patatin-like phospholipid domain containing protein; PTEN, hepatocyte-specific phosphatase and tensin homologue; SREBP, sterol regulatory-element binding protein; YY, ying yang.
| Species | Model | Characteristics | Remarks | Reference |
|---|---|---|---|---|
| Mouse | Leptin-deficient:ob/ob; db/db | - Hyperphagia - Obesity - IR - Hyperinsulinemia - Hyperglycemia - Fatty liver | - Require “second hit” for evolution to NASH | [186-188] |
| AP2diphA +/+ | - Hyperphagia - Hyperlipidemia - Hyperglycemia - IR | [189] | ||
| CD36 −/− | - Increased plasma fatty acids and TGs | [193] | ||
| MC4 −/− | - NASH - Obesity - IR - Dyslipidemia | - HFD to progress to liver fibrosis and HCC | [304] | |
| SREBP1a +/+ | - Steatosis | [190] | ||
| SREBP1c +/+ | - Hyperphagia - Steatosis - IR - Increased TG levels | [192] | ||
| PPARα −/− | - Severe steatosis | - After fasting | [305] | |
| Gal3 −/− | - Fatty changes | [306] | ||
| MTPa −/− | - Steatosis | [307] | ||
| MTPa +/− | - Steatosis - IR | [308] | ||
| AOX −/− | - Microvesicular steatosis - NASH | [309] | ||
| PTEN −/− | - Steatosis - NASH - HCC | [310] | ||
| MAT1A −/− | - Steatosis - NASH - HCC | [311] | ||
| AdipoR1 −/− | - Mild IR | [312] | ||
| AdipoR2 −/− | - Mild IR | |||
| AdipoR1 −/−/R2 −/− | - Increased TGs - Oxidative stress - Steatosis | [313] | ||
| IL6 −/− | - Obesity - Steatosis - NASH - IR | [314] | ||
| FXR −/− | - Altered lipid and cholesterol metabolism | [315] | ||
| LDLr −/− | - Macrovesicular steatosis | - Need to be fed a HFD | [315] | |
| LDLr−/−/FXR−/− | - Macrovesicular steatosis - Inflammation | [315] | ||
| PECAM1 −/− | - Microvesicular steatosis | [316] | ||
| CBS +/−CBS −/− | - Inflammation - Fibrosis - Steatosis | [317] | ||
| p62 +/+ | - Elevated hepatic cholesterol - Increased monounsaturated fatty acids | [194] | ||
| PNPLA3/148M variant | - Increased TG levels | Need to be fed a high-fructose diet | [199] | |
| ApoE2 +/+ | - Hepatic steatosis | [200] | ||
| Rat | fa/fa Zucker rat | - Obesity - Fatty liver - IR - Hyperglycemia | [318] | |
| Zebrafish | GMPS mutant | - Increased TGs | [201] | |
| CBR1 +/+ | - Lipid accumulation | [202] | ||
| YY1 +/+ | - Lipid accumulation | [203] | ||
| CDIPT −/− | - Macrovesicular steatosis - Ballooning - Necroapoptosis | [205] |
Guanosine monophosphate synthetase mutant zebrafish larvae develop liver steatosis characterized by an accumulation of lipid droplets and increased TG levels. De novo guanosine monophosphate synthesis is required to prevent hepatic steatosis and inhibition of Rac1 activity in hepatocytes induces liver steatosis [201]. Cannabinoid receptor 1-transgenic zebrafish show hepatic lipid accumulation, while the expression of sterol regulatory element-binding protein 1c, acetyl-CoA carboxylase-1 and fatty acid synthase is upregulated [202]. Moreover, ying yang 1-transgenic zebrafish present also hepatic lipid accumulation. This indicates that ying yang 1, a transcription factor belonging to the GLI-Kruppel class of zinc finger proteins, promotes lipid accumulation. Important lipogenic factors such as PPARγ, sterol regulatory element-binding protein 1c and CCAAT/enhancer-binding protein α, and key lipogenic enzymes, like acetyl-CoA carboxylase 1 and diglyceride acyltransferase, are upregulated in this steatotic transgenic zebrafish model [203]. Gankyrin, a proteasome-interacting protein, is known to be overexpressed in HCC and causes increased lipid content in the liver of transgenic zebrafish. Since hepatocellular endoplasmic reticulum stress recently has been associated with IR in diabetes, obesity and NAFLD [204], zebrafish with mutant phosphatidylinositol, being an important regulator of calcium homeostasis, membrane trafficking, secretory pathways and signal transduction, exhibit revocation of de novo phosphatidylinositol synthesis and NAFLD features in hepatocytes, characterized by macrovesicular steatosis, ballooning and necroapoptosis. In addition, an increase of endoplasmic reticulum stress response genes is observed in hi559 mutants, supporting the role of endoplasmic reticulum stress in the development of NAFLD [205].
3.2. In vitro models
3.2.1. Monolayer cultures of primary hepatocytes
Cultures of primary hepatocytes are considered the most relevant _in vivo_-like liver-based in vitro models. Primary hepatocytes are typically isolated from freshly removed liver tissue by means of the two-step collagenase perfusion technique [206]. A disadvantage of cultures of primary hepatocytes is that they progressively lose their liver-specific functionality and morphology in culture. Nevertheless, they can be of value to experimental liver steatosis research. Cultures of primary C57BL/6 mouse hepatocytes simultaneously exposed to oleic acid and palmitic acid preferably take up the former and show intracellular lipid droplets [167, 207]. When mixed, more steatosis is observed compared to oleic acid and palmitic acid alone [163, 208, 209]. Interestingly, the addition of interleukin-17, a pro-inflammatory cytokine, exacerbated the accumulation of lipid droplets in hepatocytes [207]. Isolated hepatocytes from Sprague Dawley rats accumulate lipid droplets upon incubation with VPA, amiodarone, tetracycline or tamoxifen [210]. The use of primary human hepatocytes faces many challenges, including availability, phenotypic instability and limited lifespan [211]. Moreover, triglyceride accumulation causes a downregulation of CYP enzymes in cultures of human primary hepatocytes, which may affect the metabolism of administered drugs [212]. Nevertheless, human hepatocytes supplemented with oleic acid or eicosapentaenoic acid have an increased presence of TG-based lipid droplets and VLDL secretion without a change in apolipoprotein B abundance [213], which makes them a suitable in vitro model for the study of liver steatosis.
3.2.2. Monolayer cultures of liver-based cell lines
Hepatocarcinoma-derived cell lines present some advantages and disadvantages over primary hepatocytes. Their high availability, easy handling and unlimited life span make cell lines attractive models. However, genetic instability and a phenotype that strongly differs from that of primary hepatocytes are drawbacks of these systems [214]. HepG2 cells originate from human HCC and retain several biochemical functions of liver parenchymal cells [215], including the potential to secrete lipoproteins [216]. Consequently, the HepG2 cell line is a suitable model for studying human lipid metabolism. HepG2 cells exposed to different concentrations of oleic acid or palmitic acid exhibit intracellular accumulation of lipid droplets and TGs [163, 168-170, 208, 217]. Similarly, tamoxifen causes an increase of lipid droplets in the cytosol of HepG2 cells [218]. It should be mentioned, however, that HepG2 cells lack CYP activity, important for xenobiotic metabolism. Therefore, a number of subclones, such as HepG2/C3A cells, have been developed, which express functional CYP activity and that display accumulation of TGs after exposure to oleic acid either alone or combined with palmitic acid [219]. The HepaRG cell line stems from a female suffering from HCC. When seeded at low density, HepaRG cells quickly recover bipotent progenitors and actively divide until they reach confluence. Subsequently, they differentiate into hepatocyte-like and biliary-like cells. HepaRG cells express major liver-specific functions, including CYP activity, are functionally stable at confluency and have an indefinite growth potential [211]. HepaRG cells have great potential in in vitro liver steatosis research, as they accumulate lipid droplets following exposure to stearic acid, palmitic acid, oleic acid, linoleic acid, eicosapentaenoic acid, docosahexaenoic acid and amiodarone [220, 221]. This also holds true for Huh-7 cells, a well-differentiated HCC cell line, which is able to pile up lipid droplets when treated with oleic acid either alone or together with palmitic acid [163, 222, 223].
3.2.3. Co-cultures
Culturing hepatocytes and non-parenchymal cells together modulates cell growth and hepatocyte morphology and increases functionality [224]. Also, co-cultures of hepatocytes with Kupffer cells or fibroblasts provide improved hepatocyte viability and function [225, 226]. Co-cultures of Huh-7 cells and LX-2 hepatic stellate cells, exposed to a mixture of oleic acid and palmitic acid, were found to accumulate intracellular fat. In these co-cultures, stellate cells became activated, assessed by alpha-smooth muscle actin expression. Furthermore, a time-dependent increase of tissue inhibitor metalloproteinase-2 protein has been observed, suggesting enhanced collagen biosynthesis [227]. Similarly, matrix-metallo-proteinase-2 and other profibrogenic genes, such as tissue inhibitor of metallo-proteinase-1 and transforming growth factor β were upregulated in a co-culture of primary human hepatocytes, which were treated with palmitic acid, and human hepatic stellate cells [228]. Micropatterned co-cultures allow the development of heterogeneous surfaces on which control over cell-cell interactions is possible in the micrometer range. This enables modulation of the phenotype of liver cells in vitro [229]. Co-cultures of primary human hepatocytes and murine embryonic fibroblasts have shown to maintain morphology, gene expression and functionality up to six weeks [230]. Additionally, the use of micropatterned co-cultures delivers a higher sensitivity in the prediction of drug toxicity outcomes in comparison with conventional twodimensional cultures [231]. In this context, a microscale model of primary human hepatocytes and stromal fibroblasts was established and proved to be a robust model for the study of glucose metabolism, which is closely associated with NAFLD. This was possible for several weeks and thus would allow the study of the effects of drugs in diabetes [232].
3.2.4. Sandwich cultures
To partly overcome the rapid decline in functionality and morphology of primary hepatocytes in monolayer culture established in plastic dishes, sandwich cultures are often used. Here, primary hepatocytes are cultivated in a confluent monolayer between two layers of collagen type one [233]. This set-up decreases the functional decline and allows the application of a more time-consuming experimental set-up [234]. Hepatocytes isolated from lean Zucker rats cultivated in a collagen sandwich configuration developed microvesicular and macrovesicular steatosis upon exposure to a mix of oleic acid and linoleic acid after three and six days, respectively [235]. Additionally, primary human hepatocytes in sandwich culture treated with chlorpromazine showed changes in the genetic signature related to fibrosis, inflammation, necrosis and steatosis after short-term and long-term chlorpromazine treatment [236]. Primary rat hepatocytes, cultured between two layers of collagen, showed lipid droplets following incubation with VPA, amiodarone, tetracycline or tamoxifen [237].
3.2.5. Spheroid cultures
Planar culture systems do not represent the complex architecture of hepatic tissue in vivo. Culture of hepatocytes into spheroids may improve some of their functions due to the establishment of cell-cell contacts and presence of extracellular matrix components within and around the aggregates [234]. Today, the use of spheroid models is limited in experimental NAFLD research. H35 rat hepatoma cells in spheroid configuration have been exposed to free fatty acids, leading to increased fatty acid uptake and increased intracellular TG accumulation. In turn, this decreased DNA content, reduced proliferation and increased intracellular reactive oxygen species [238]. Oleic acid-treated HepG2 spheroids have also been proven useful in vitro tools for studying hepatic steatosis. These spheroids were surprisingly less susceptible to cytokine and pro-oxidant damage via an adaptive mechanism dependent on adenosine monophosphate-activated protein kinase activity [239]. Additionally, a spheroid model of primary hepatocytes from male Sprague Dawley rats shows higher intracellular glycogen content, glucose consumption, and gluconeogenesis, and a more _in vivo_-like sensitivity to insulin and glucagon in comparison with monolayer cultures. Finally, the exposure of primary hepatocytes to high levels of glucose induced toxicities, including alteration of mitochondrial membrane potential, lipid accumulation and reactive oxygen species formation [240].
3.2.6. Precision-cut liver slices
Precision-cut mice liver slices can act as valuable tools for the study of lipid metabolism, especially since they are considered to closely reflect the in vivo situation. In these systems, all cell types are present, thus maintaining overall cell-cell and cell-extracellular matrix interactions [241]. Although liver slices are promising in vitro models, they have not been used extensively in NAFLD research [242]. Liver slices exposed to VPA, amiodarone and tetracycline have been used to identify genes altered by steatogenic drugs. Microarray analysis shows that amiodarone and VPA upregulate lipid metabolism, whereas processes associated with extracellular matrix remodeling and inflammation were downregulated. Tetracycline causes a downregulation of mitochondrial functions, lipid metabolism and fibrosis [243]. Liver slices from ob/ob mice showed a reduced VLDL secretion in CD36-deficient mice, which highlights the importance of CD36 and VLDL in the development of NAFLD [244].
4. Biomarkers in NAFLD research
4.1. Epigenetic biomarkers
Genes involved in lipid and glucose metabolism, development of fibrosis and liver tissue remodeling are known to be regulated by DNA methylation [245]. Thus, DBA/2J mice fed a lipogenic methyl-deficient diet show DNA demethylation in liver, which is less pronounced compared to C57BL/6J counterparts. This is associated with a loss of cytosine methylation at repeated sequences, such as major and minor satellites, long interspersed nuclear elements, short interspersed nuclear elements and intracisternal A-particle elements [142]. Significantly altered genes encoding chromatin-remodeling enzymes, including jumonji C-domain-containing histone demethylases, which regulate histone H3K9 and H3K4 trimethylation, have been observed in livers of steatotic hAPOE2 mice. This is accompanied by modified expression of genes related to PPARα and hepatic lipid catabolism [246]. In NASH patients, mitochondrial nicotinamide adenine dinucleotide dehydrogenase 6 gene methylation was found to be increased, whereby the degree of methylation was linked to the severity of NAFLD. This goes hand in hand with decreased production of mitochondrial nicotinamide adenine dinucleotide dehydrogenase 6 and increased DNA methyltransferase I expression [247]. The DNA methylation status of the PPAR c coactivator 1α gene positively correlates with plasma fasting insulin levels and IR in NAFLD patients, while the inverse holds true for the mitochondrial transcription factor A gene [248]. In the last few years, circulating microRNAs (miRs) gained quite some interest as possible diagnostic biomarkers of NAFLD.
MiR-122, an miR species highly expressed in liver, was shown to be an important regulator in cholesterol metabolism [249], HCC [250] and hepatitis C infection [251]. MiR-122 expression is downregulated in NASH patients [252, 253], partly evidenced by the elevated production of some of the miR-122 targets, such as sterol regulatory element-binding protein 1c, fatty acid synthase and 3-hydroxy-3-methyl-glutaryl-CoA reductase [252]. By contrast, serum levels of miR-122, miR-21, miR-34a and miR-451, all which control liver cholesterol and fatty acid homeostasis, were found to be increased in NAFLD patients, with miR-122 levels being associated with liver steatosis [254, 255]. MiR-122 knock-out mice exhibit accumulation of TGs caused by upregulation of enzymes involved in the synthesis and storage of TGs in the liver. These animals display inflammation, fibrosis and HCC [256]. Furthermore, a wide array of miRs is altered in animal models of NAFLD. In this regard, miR-29 (i.e. a potential therapeutic target in NAFLD in mice) [257], miR-34a (i.e. a regulator of apoptosis), miR-155 (i.e. a tumor suppressor regulator) and miR-200b (i.e. targets transcriptional repressors of E-cadherin) are positively affected upon feeding of C57BL/6J and DBA/2J mice a methyl-deficient diet [258]. Along the same line, downregulation of miR-122, miR-451 and miR-27 and upregulation of miR-200a, miR-200b and miR-429 occur in steatotic Sprague Dawley rats [259]. In HFD-fed Sprague Dawley rats, downregulation of enhancer of zeste homolog 2, a protein which controls the epigenetic silencing of specific genes and/or miRs by trimethylating lysine27 on histone H3, is inversely correlated with lipid accumulation [260]. Hepatic miR-34a levels are elevated in dietary and leptin-deficient ob/ob obese mice [261, 262]. MiR-34a plays a role in the regulation of gene encoding silent mating type information regulation 2 homolog, a nicotine adenine dinucleotide-dependent deacetylase that is implicated in the prevention of many age-related diseases, including metabolic disorders associated with steatosis, inflammation and glucose intolerance [263].
4.2. Genetic biomarkers
Genome-wide association studies have been instrumental for the detection of specific genetic changes in the pathology of NAFLD. These studies equally allowed the identification of steatosis biomarkers across genomes. A mutation in the patatin-like phospholipase domain containing family member A3, also named adiponutrin, on chromosome 22 is strongly associated with increased hepatic fat levels and hepatic inflammation in steatosis patients [264]. This mutation is phenotypically associated with steatosis, portal inflammation, lobular inflammation, Mallory-Denk bodies and fibrosis [265] as well was with HCC [266] in NAFLD patients. Recently, a single nucleotide polymorphism in transmembrane 6 superfamily member 2, a gene of unknown function on chromosome 19, has been linked to NAFLD [267]. It is well-known that Toll-like receptor 4 plays an important role in the pathogenesis of NAFLD. Interestingly, allelic variants of Toll-like receptor 4 (i.e. Asp299Gly and Thr399IleTLR4) in humans may prevent NAFLD, since the number of subjects with the heterozygous mutation (i.e. Asp299Gly) is significantly lower in NAFLD patients than in the control group [268]. Additionally, sterol regulatory element-binding factor 1c polymorphism reflects an increased risk of developing NAFLD with more severe liver histology and derangement in glucose and lipoprotein metabolism [269]. A common variant in the glucokinase regulatory gene and MTP-493G> T polymorphism was found to be associated with a higher NAFLD incidence [270, 271], along with more severe liver fibrosis and higher serum TG levels [272]. Furthermore, thymine-cytosine, cytosine-cytosine and thymine-thymine genotypes of apolipoprotein C3 (i.e. -455T>C) polymorphism are more susceptible to NAFLD. The cytosine-cytosine genotype is more prone to IR than the thymine-thymine genotype, while the cytosine-cytosine genotype presents a significantly higher risk of hypertension, hypertriglyceridemia and low levels of high-density lipoprotein [273].
4.3. Transcriptomic biomarkers
Transcriptomics studies the expression level of mRNAs at genome-wide level. This enables characterization of transcriptomic signatures of specific diseases. The major tool in transcriptomics is the microarray [274]. By using this technology, genes related to fatty acid metabolism, such as CYP4A14, and to cell proliferation and differentiation, like fibroblast growth factor 21 and CYP4A10, have been found upregulated in VPA-administered ICR mice, while the genes encoding glucose-6-phosphatase and protein phosphatase 2, regulatory subunit B, δ, a protein involved in cell growth are downregulated [47, 176]. Similarly, tamoxifen-treated C57BL/6 mice display hepatic transcriptional upregulation of androgen receptor, nuclear receptor subfamily 2 group F member 1, hepatocyte nuclear factor α 4 and retinoic acid receptor-related orphan receptor 1 α all related to lipid and carbohydrate metabolism [85]. Furthermore, male ICR mice exposed to tetracycline show overexpression of several genes involved in phospholipid metabolism, including choline kinase α, elongation of very long chain fatty acids, insulin-induced gene 2 and genes related to carbohydrate metabolism, such as protein phosphatase 1, regulatory subunit 3C, and prostaglandin D2 synthase [71]. Transcriptomics analysis of liver tissue of apolipoprotein E-deficient mice fed a Western-type diet enriched with linoleic acid reveals an upregulation of lymphocyte antigen 6D, genes associated with adipocyte differentiation, like fat-specific protein 27, genes related to fatty acid metabolism, such as stearoyl-CoA desaturase-1 and CD36, and a concomitant downregulation of genes involved in bile acid biosynthesis, such as CYP7B1 (cytochrome P450, 7b1) as well as genes related to steroid hormone biosynthesis, like3-ketosteroid reductase [275]. Decorin, a proteoglycan produced by monocytes and macrophages at sites of inflammation, is known to be upregulated in adipose tissue of obese human patients. This suggests a role of decorin in the development of inflammation in NASH. This is further substantiated by the observation that decorin production is increased in the liver of ob/ob, db/db and C57BL/6J mice fed a HFD. Other upregulated genes include endothelial membrane protein 1, a peripheral myelin protein suggested to modulate cell-matrix and cell-cell interactions, and IκB kinase β-interacting protein, which links the transcription factor NF-κB to the apoptotic signaling pathway in the endoplasmic reticulum. Reduction of the former decreases cytokine expression, suggesting a protective role against IR [276]. Protein tyrosine phosphatases fulfill an important function in regulating insulin action and hepatic glucose metabolism protein tyrosine phosphatase 6 knock-out mice fed a HFD are protected from IR, but are more prone to hepatic steatosis and show an upregulation of the PPARγ gene [277]. Liver tissue of human liver steatosis patients presents enhanced gene expression of evolutionarily conserved signaling intermediate in Toll pathway (i.e. an adapter protein of the Toll-like and interleukin-1 receptor signaling pathway involved in the assembly of mitochondrial nicotinamide adenine dinucleotide), Toll-interacting protein and single immunoglobulin interleukin-1-related molecule. This overexpression can be attributed to the activation of Kupffer cells or hepatocytes releasing interleukins that act on hepatic stellate cells [278]. mRNA quantities of keratin type I cytoskeletal 23, a protein related to metabolism, and aldo-keto reductase family 1 member B10, a protein involved in the control of cytoarchitecture, are upregulated in steatohepatitis compared to steatosis and normal liver, and hence have been proposed as potential biomarkers for steatohepatitis as well as for HCC progression [279]. An overview of transciptomics biomarkers in models of liver steatosis is displayed in Table 2.
Table 2. Transcriptomic biomarkers of NAFLD.
CoA, coenzyme A; CYP, cytochrome P450; CD36, cluster of differentiation; HFD, high-fat diet; PPAR, peroxisome proliferator-activated receptor; PTPN, tyrosine-protein phosphatase non-receptor; VPA, valproic acid.
| Species | Upregulated genes | Downregulated genes | Reference | |
|---|---|---|---|---|
| Human | Steatotic liver | - Single immunoglobulin domain-containing IL1R-related molecule - Likely ortholog of mouse signaling intermediate in Toll pathway-evolutionarily conserved - Toll interacting protein | [253] | |
| Steatotic liver | - Aldo-keto reductase family 1 member B10 - Keratin, type I cytoskeletal 23 | [254] | ||
| Mouse | VPA-induced liver steatosis | - CYP4A14 - CYP4A10 | - Glucose-6-phosphatase - Protein phosphatase 2, regulatory subunit B, δ | [47] |
| VPA-induced liver steatosis | - CYP4A14 - Fibroblast growth factor 21 - Glutathione S-transferase A2 | - Flavin containing monooxygenase 3 | [176] | |
| Tamoxifen-induced liver steatosis | - Androgen receptor - Nuclear receptor subfamily 2 group F member 1 - Hepatocyte nuclear factor α 4 - Retinoic acid receptor-related orphan receptor 1 α | [85] | ||
| Linoleic acid isomer-induced liver steatosis in ApolipoproteinE-deficient mouse | - Lymphocyte antigen 6D - Fat-specific protein 27 - Aquaporin 4 - Uridine kinase 1 - Stearoyl-CoA desaturase-1 - CD36 | - CYP7B1 - Trefoil factor 3 intestinal - Synaptotagmin 1 - 3-ketosteroid reductase | [250] | |
| Tetracycline-induced liver steatosis in mouse | - Choline kinase α - CYP2A12 - CYP2C38 - Elongation of very long chain fatty acids - Phosphatidylinositol-4-phosphate 3-kinase, catalytic subunit type 2 γ - Insulin induced gene 2 - Protein phosphatase 1, regulatory subunit 3C) - Prostaglandin D2 synthase 21kDa | [71] | ||
| Liver of C57BL/6J mice fed a HFD, ob/ob mice and db/db mice | - Decorin - Epithelial membrane protein 1 - IκB kinase β interacting protein | [251] | ||
| Liver of Ptpn6 knock-out mice | - PPARγ | [252] |
4.4. Proteomic biomarkers
Compared to the genome, the proteome can provide a more dynamic and accurate reflection of both the intrinsic genetic program of the cell and the impact of environmental factors. Gene expression changes do not always accurately predict protein levels because proteins can undergo posttranslational modifications, including phosphorylation and glycosylation [280]. Proteomics techniques, such as tandem liquid chromatography-mass spectrometry and two-dimensional gel electrophoresis, can be used to study changes in proteins at large-scale, in casu in vitro and in vivo models of liver steatosis (see Table 3). Using such approaches, it has been found that hamsters fed a high-fructose diet abundantly produce many proteins, including fatty acid binding protein, carbamoyl-phosphate synthase, apolipoprotein A1, protein disulfide isomerase, proteins involved in anti-oxidation, such as peroxiredoxin 2 and heat shock protein 70, fructose-1,6-biphosphatase and glycerol kinase [281]. Upon feeding male C57BL/6J mice a HFD enhanced protein production of cytokeratine 8 and 18, vimentin and apolipoprotein E, but decreased quantities of glutathione peroxidase are observed [282]. Furthermore, major urinary protein 2 is overexpressed in apolipoprotein E-null mice. It has been suggested that this may represent a compensatory, protective response triggered in the liver. In turn, this could play a role in plasma lipid homeostasis, since another subunit of major urinary protein 2, namely major urinary protein 1, enhances mitochondrial biogenesis and decreases lipid accumulation in the liver of db/db mice [283]. Also, mitochondrial antioxidant expression is increased in apolipoprotein E-null mice, in particular peroxiredoxin-4, thioredoxin-dependent peroxide reductase and glutathione peroxidase 1, due to an increase in reactive oxygen species [284]. Liver tissue of female C57BL/6N mice fed a HFD exhibits increased protein expression of methylenetetrahydrofolate dehydrogenase 1, while translational levels of glucose transporter 1, methylthioadenosine phosphorylase and methionine adenosyltransferase 1α are decreased [285]. Likewise, db/db mice show a number of overexpressed proteins, including annexin 5 (i.e. a well-known apoptotic marker), cadherin 2 (i.e. a protein involved in insulin signaling), transporter 2, 24-dehydrocholesterol reductase (i.e. a protein related to inflammation and possibly to insulin signaling) and guanosinetriphosphate-binding protein SAR1 gene homolog B, whereas other are underexpressed, such as epidermal growth factor, six-transmembrane epithelial antigen of prostate 4 and translocation protein SEC62 [286]. Heat shock proteins are significantly downregulated in liver of rats fed HFD [287]. Such steatosis-related protein changes have also been observed in human patients. Thus, fatty acid binding protein 1 is overexpressed in livers of steatosis patients, but is downregulated during different stages in NASH progression [288]. Moreover, protein analysis of lipid droplets in human steatotic liver samples revealed a yet unidentified lipid droplet-associated protein which is upregulated upon fat accumulation. Interestingly, its protein expression has been validated in db/db mice and HFD-fed mice [289].
Table 3. Proteomic biomarkers of NAFLD.
HFD, high-fat diet.
| Species | Validated upregulated proteins | Validated downregulated proteins | Reference | |
|---|---|---|---|---|
| Human | Liver of steatosis patients | - Fatty acid binding protein | [263] | |
| Liver of steatosis patients | - 17β-HSD13 | [264] | ||
| Hamster | Liver of hamsters fed a fructose diet | - Fatty acid binding protein - Apolipoprotein A1 - Protein disulfide isomerase | - Heat shock protein 70 - Senescence marker - Protein 30 - Chaperonin GroEL | [256] |
| Mouse | Liver of mice fed a HFD | - Cytokeratin 8 - Cytokeratin 18 - Vimentin - Apolipoprotein E | - S-adenosylhomocysteine hydrolase | [257] |
| Liver of ApolipoproteinE-knock-out mice | - Major urinary protein 2 | - Glutathione peroxidase 1 | [259] | |
| Liver of mice fed a fat-sucrose diet | - Heat shock protein 70 | [262] | ||
| Liver of mice fed a cholesterol diet | - Methylenetetrahydrofolate dehydrogenase 1 - Lactate dehydrogenase A | - Glucose transporter 1 - Methylthioadenosine - Phosphorylase - Methionine - Adenosyltranferarse 1α | [260] | |
| Liver of db/db mice | - Annexin A5 - Dehydrocholesterol reductase - transporter 2 - Cadherin 2 - Guanosinetriphosphate-binding protein SAR1b | - Epidermal growth factor receptor - Six-transmembrane epithelial antigen of prostate 4 - Translocation protein SEC62 | [261] |
4.5. Metabonomic biomarkers
Among all ‘-omics’, metabonomics is the most stable, most closely represents the phenotype and most appropriately mirrors the activities of the cell. The main methodologies used for metabonomics analysis are mass spectrometry and nuclear magnetic resonance spectroscopy [290]. By applying such techniques, it has been found that PPARα knock-out mice exhibit a decrease in glucose and glycogen content, and a concomitant increase in linoleic acid, oleic acid and di-homo-y-linolenic acid in liver [291]. In a similar study, an increase in TGs, free and esterified cholesterol, oleic acid, leucine, valine, lysine and methionine and a decrease in arachidonic acid, eicosapentaenoic acid, docosahexaenoic acid and carnitine were observed in liver of low density lipoprotein knock-out mice fed a cholesterol-rich diet [292]. Rats administered aflatoxinB1 develop hepatic steatosis associated with elevated levels levels of glucose, amino acids, choline, phosphocholine, and glycerophosphocholine, and decreased lipid amounts in blood. Simultaneously, aflatoxinB1 increases quantities of lipids, tyrosine, histidine, phenylalanine, leucine, isoleucine, valine, choline, inosine, adenosine, and uridine, but decreases glycogen and glucose in liver [293]. HFD-fed C57BL/6J mice show high levels of oxidized glutathione, choline, taurine, sarcosine and downregulated amounts of reduced glutathione, methionine, serine, glycine, ethanolamine and phosphoethanolamine [294]. When these animals additionally receive leucine through drinking water, a marked improvement in hepatic steatosis, inflammation in adipose tissue, glucose tolerance and insulin signaling was noticed, suggesting that leucine acts as an important future adjunct in the management of obesity-related IR [295]. The liver of paraoxonase-1-deficient mice fed a HFD contains high levels of methionine sulfoxide, taurine, fructose, glucose and 1,3-dihydroxyacetone, but low levels of glycine, glutamate, cysteine, hypotaurine, methionine, homocysteine, glutathione, 3-phospoglycerate, phosphoenolpyruvate, lactate, citrate, _cis_-aconiate, succinylcarnitine, fumarate, malate and phosphate [296]. In serum of male C57BL/6NCr mice fed an MCD diet, abundant e oleic acid, linoleic acid, non-esterified fatty acids, tauro-β-muricholate, taurocholate and 12-hydroxyeicosatetraenoic acid and low production of stearoyllysophosphatidylcholine, oleoyllysophosphatidylcholine and palmitoyllysophosphatidylcholine have been described, all which points to alterations in phospholipid and bile acid metabolism [297, 298]. Similarly, taurochendeoxycholate and taurocholate are upregulated, while glycochendeoxycholate and glycocholate are downregulated in the serum of tetracycline-treated male C57BL/6NCr mice. These findings indicate that perturbation of bile acid homeostasis is an early event in drug-induced liver steatosis and that these metabolites may represent sensitive and early biomarkers for diagnosis of liver steatosis [299]. Four metabolites in the liver of mice fed a HFD are also altered in liver samples of steatosis and NASH patients, namely glucose, glutamate/glutamine, lactate and taurine, which again suggests a role as clinically relevant read-outs [300]. Metabolites upregulated in human liver steatosis and NASH patients include glutamyl dipeptides, glutamyl valine, glutamyl leucine, glutamyl phenylalanine, glutamyl tyrosine, free carnitine, butyrylcarnitine, glutamate, lysine, tyrosine and isoleucine, whereas cysteine-glutathione disulfide, caprate, 10-undecenoate and 1-oleoylglycerophosphocholine are downregulated [301]. Moreover, human NASH livers exhibit increased levels of taurine, taurocholic acid and taurodeoxycholic acid with concomitant decreased presence of cholic acid and glycodeoxycholic acid [302]. In addition, increased production of glycerophosphocholine, glycerylphosphorylethanolamine, taurine and glycine conjugates were found in liver tissue of steatosis patients, indicating disturbances in lipid and bile acid homeostasis and mitochondrial dysfunction. Furthermore, these patients also display elevated levels of hypoxanthine, creatinine, glutamate, glutamine, glutathione and ATP, suggesting alterations in energy metabolism and amino acid metabolism [303]. A detailed summary of metabonomic biomarkers in models of liver steatosis is shown in Table 4.
Table 4. Metabonomic biomarkers of NAFLD.
HFD, high-fat diet; MCD, methionine-choline-deficient diet; PC, phosphatidylcholine; PPAR, peroxisome proliferator-activated receptor.
| Species | Upregulated metabolites | Downregulated metabolites | Reference | |
|---|---|---|---|---|
| Human | Liver from steatosis patients | - Glutamyl dipeptides - Glutamyl valine - Glutamyl leucin - Glutamyl phenylalanine - Glutamyl tyrosine - Carnitine - Butyrylcarnitine - Glutamate - Lysine - Tyrosin - Isoleucine | - Cysteine-glutathione disulfide - Caprate - 10-undecenoate - OleoyllysoPC | [276] |
| Liver of steatosis patients | - GlyceroPC - Glycerylphosphorylethanolamine - Taurine - Glycine conjugates | - Oxidized and reduced glutathione - Glutamate - Adenosine triphosphate - Creatine | [278] | |
| Liver of NASH patients | - Taurine - Taurocholic acid - Taurodeoxycholic acid | - Cholic acid - Glycodeoxycholic acid | [277] | |
| Serum of steatosis patients | - Lactate - Glutamate - Taurine | [275] | ||
| Mouse | Plasma of rosiglitazone-treated mice | - Phosphatidylcholine - Triacylglyceride - Cholesterol - Leptin - Insulin - Glucose | [298] | |
| Liver of mice subjected to starvation | - Free cholesterol - Triacylglycerols - Cholesterol esters | - PC | [299] | |
| Liver of PPARα knock-out and age-related steatotic mice | - Linoleic acid - Oleic acid - Di-homo-γ-linolenic acid | - Glucose - Glycogen | [266] | |
| Liver of LDLr knock-out mice fed a cholesterol-rich diet | - Triglycerides - Free and esterified cholesterol - Oleic acid - Leucine - Valine - Lysine - Methionine | - Arachidonic acid - Eicosapentaenoic acid - Docosahexaenoic acid - Carnitine | [267] | |
| Liver of mice fed a HFD | - Oxidized glutathione - Choline - Taurine - Sarcosine | - Reduced glutathione - Methionine - Serine - Glycine - Ethanolamine - Phosphoethanolamine | [269] | |
| Serum of mice fed a MCD | - Lactate - Glutamate - Taurine | [275] | ||
| Liver of mice fed a HFD | - Fructose - Sorbitol - α-hydroxybutyrate | - Leucine - Fumarate - Malate - Cholesterol - Nicotinamide adenine dinucleotide - Tryptophan | [270] | |
| Serum of mice fed a MCD | - Tauro-β-muricholate - Taurocholate - 12-hydroxyeicosatetraenoic acid | - StearoyllysoPC - OleoyllysoPC - PalmitoyllysoPC | [272] | |
| Liver of paraoxonase-1-deficient mice fed a HFD | - Methionine sulfoxide - Taurine - Fructose - Glucose - 1,3-dihydroxyacetone | - Glycine - Glutamate - Cysteine - Hypotaurine - Methionine - Homocysteine - Glutathione - 3-phospoglycerate - Phosphoenolpyruvate - Lactate - Citrate - Cis-aconiate - Succinylcarnitine - Fumarate - Malate - Phosphate | [271] | |
| Serum of mice fed a MCD | - Oleic acid - Linoleic acid - Non-esterified fatty acid | [273] | ||
| Plasma of mice of tetracycline-induced steatosis | - Taurochendeoxycholate - Taurocholate | - Glycochendeoxycholate - Glycocholate | [274] | |
| Rat | Liver of aflatoxin B1-treated rats | - Lipids - Tyrosine - Histidine - Phenylalanine - Leucine - Isoleucine - Valine - Choline - Inosine - Adenosine - Uridine | - Glucose - Glycogen | [268] |
5. Conclusions and perspectives
As evidenced by epidemiological studies, NAFLD is currently a worldwide health problem [1, 13]. Hepatic steatosis, the first stage in the NAFLD spectrum, is related to type 2 diabetes mellitus, obesity and drug-induced liver injury [2, 304]. At present, histopathological examination of liver tissue is considered the golden standard for diagnosing. This procedure requires biopsy, which is not without risk. In fact, the ideal diagnostic biomarker of hepatic steatosis would be a non-invasive marker suitable for early detection of the disease and able to reflect the severity of the disease [305]. Great promise lies with metabonomic, proteomic and transcriptomic biomarkers, as they provide mechanistic information. In a similar way, the ideal liver steatosis model should fully reproduce the human pathology. To date, however, not a single in vivo model encompasses the spectrum of human disease progression. Rather, these models imitate particular characteristics of human NAFLD. Among those, the HFD model still most closely resembles clinical NAFLD, as it is associated with obesity, IR and type 2 diabetes mellitus as well as with a human-relevant histopathological pattern in liver [306]. In vitro models, especially cultures of primary hepatocytes and hepatic cell lines, are also valuable in liver steatosis research. Nevertheless, a combination of different models could be the most effective strategy in NAFLD research. Given the blossoming of stem cell-based in vitro models in the last few years, it can be expected that hepatocyte-like cells generated from stem cells will join in as in vitro liver steatosis test systems in near future [307]. It is of critical importance that researchers are aware of the power, but especially the shortcomings, of the experimental models in order to ensure scientifically sound conclusions and appropriate extrapolation to the human situation. When appropriately used, these models, together with the various drug-based and diet-based strategies as well as with the relevant read-outs for evaluation, will continue to be indispensable tools in liver steatosis research. This, in turn, will undoubtedly result in new clinical approaches for the diagnosis and therapy of liver steatosis and NAFLD.
Acknowledgments
This work was financially supported by the grants of the University Hospital of the Vrije Universiteit Brussel-Belgium (Willy Gepts Fonds UZ-VUB), the Fund for Scientific Research-Flanders (FWO grants G009514N and G010214N), the European Research Council (ERC Starting Grant 335476), the University of São Paulo-Brazil (USP) and the Foundation for Research Support of the State of São Paulo (FAPESP SPEC grant 2013/50420-6).
Glossary
ACS
acyl-coenzyme A synthase
adipoR
adiponectin receptor
ADP
adenosine diphosphate
AP2diph
attenuated P2 diphtheria toxin
AOX
acyl-coenzyme A oxidase
ApoE
apolipoprotein E
ATP
adenosine triphosphate
CBS
cystathionine-β-synthase
CD36
cluster of differentiation 36
CoA
coenzyme A
CPT1
carnitine palmitoyl transferase 1
CYP
cytochrome P450
DNA
deoxyribonucleic acid
FAD
oxidized flavin adenine dinucleotide
FADH2
reduced flavin adenine dinucleotide
FFAs
free fatty acids
FXR
farnesoid x receptor
Gal
galectin
HCC
hepatocellular carcinoma
HFD
high-fat diet
IL-6
interleukine-6
IMS
intermembrane space
IR
insulin resistance
LDL
low density lipoprotein
LDs
lipid droplets
MA
macrovesicular steatosis
MAT
methionine adenosyl transferase
MC4
melanocortin 4
MCD
methionine and choline-deficient diet
MI
microvesicular steatosis
(micro)RNA
(micro)ribonucleic acid
MPT
mitochondrial permeability transition
MRC
mitochondrial respiratory chain
MTP
mitochondrial trifunctional protein
MTTP
mitochondrial triglyceride transfer protein
NAD
oxidized nicotinamide adenine dinucleotide
NADH
reduced nicotinamide adenine dinucleotide
NAFLD
non-alcoholic fatty liver disease
NASH
non-alcoholic steatohepatitis
PC
phosphatidylcholine
PECAM
platelet endothelial cell adhesion molecule
PNPLA
patatin-like phospholipid domain containing protein
PPAR
peroxisome proliferator-activated receptor
PTEN
hepatocyte-specific phosphatase and tensin homologue
SREBP
sterol regulatory-element binding protein
TGs
triglycerides
VLDL
very low density lipoproteins
VPA
valproic acid
Footnotes
Authors’ declaration of personal interests
The authors report no conflict of interest or personal interest.
References
- [1].Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- [2].Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274–85. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- [3].Milić S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis. 2012;30:158–62. doi: 10.1159/000336669. [DOI] [PubMed] [Google Scholar]
- [4].Bedogni G, Bellentani S. Fatty liver: how frequent is it and why? Ann Hepatol. 2004;3:63–5. [PubMed] [Google Scholar]
- [5].Bellentani S, Bedogni G, Miglioli L, Tiribelli C. The epidemiology of fatty liver. Eur J Gastroenterol Hepatol. 2004;16:1087–93. doi: 10.1097/00042737-200411000-00002. [DOI] [PubMed] [Google Scholar]
- [6].Björnsson E. The natural history of drug-induced liver injury. Semin Liver Dis. 2009;29:357–63. doi: 10.1055/s-0029-1240004. [DOI] [PubMed] [Google Scholar]
- [7].Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28(Suppl 1):68–76. doi: 10.1111/jgh.12212. [DOI] [PubMed] [Google Scholar]
- [8].Obika M, Noguchi H. Diagnosis and evaluation of nonalcoholic fatty liver disease. Exp Diabetes Res. 2012;2012:145754. doi: 10.1155/2012/145754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lebovics E, Rubin J. Non-alcoholic fatty liver disease (NAFLD): why you should care, when you should worry, what you should do. Diabetes Metab Res Rev. 2011;27:419–24. doi: 10.1002/dmrr.1198. [DOI] [PubMed] [Google Scholar]
- [10].Adams LA, Feldstein AE. Non-invasive diagnosis of nonalcoholic fatty liver and nonalcoholic steatohepatitis. J Dig Dis. 2011;12:10–6. doi: 10.1111/j.1751-2980.2010.00471.x. [DOI] [PubMed] [Google Scholar]
- [11].Tannapfel A, Denk H, Dienes HP, Langner C, Schirmacher P, Trauner M, et al. Histopathological diagnosis of non-alcoholic and alcoholic fatty liver disease. Virchows Arch. 2011;458:511–23. doi: 10.1007/s00428-011-1066-1. [DOI] [PubMed] [Google Scholar]
- [12].Moroşan E, Mihailovici MS, Giuşcă SE, Cojocaru E, Avădănei ER, Căruntu ID, et al. Hepatic steatosis background in chronic hepatitis B and C - significance of similarities and differences. Rom J Morphol Embryol. 2014;55:1041–7. [PubMed] [Google Scholar]
- [13].Blachier M, Leleu H, Peck-Radosavljevic M, Valla DC, Roudot-Thoraval F. The burden of liver disease in Europe: a review of available epidemiological data. J Hepatol. 2013;58:593–608. doi: 10.1016/j.jhep.2012.12.005. [DOI] [PubMed] [Google Scholar]
- [14].Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134–44. doi: 10.1053/j.gastro.2009.02.038. [DOI] [PubMed] [Google Scholar]
- [15].Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824–38. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]
- [16].Corey KE, Kaplan LM. Obesity and liver disease: the epidemic of the twenty-first century. Clin Liver Dis. 2014;18:1–18. doi: 10.1016/j.cld.2013.09.019. [DOI] [PubMed] [Google Scholar]
- [17].van Agthoven M, Metselaar HJ, Tilanus HW, de Man RA, IJzermans JN, Martin van Ineveld BM. A comparison of the costs and effects of liver transplantation for acute and for chronic liver failure. Transpl Int. 2001;14:87–94. doi: 10.1007/s001470050852. [DOI] [PubMed] [Google Scholar]
- [18].Tauchi-Sato K, Ozeki S, Houjou T, Taguchi R, Fujimoto T. The surface of lipid droplets is a phospholipid monolayer with a unique Fatty Acid composition. J Biol Chem. 2002;277:44507–12. doi: 10.1074/jbc.M207712200. [DOI] [PubMed] [Google Scholar]
- [19].Burt AD, Mutton A, Day CP. Diagnosis and interpretation of steatosis and steatohepatitis. Semin Diagn Pathol. 1998;15:246–58. [PubMed] [Google Scholar]
- [20].Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–5. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- [21].Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol. 2002;16:663–78. doi: 10.1053/bega.2002.0333. [DOI] [PubMed] [Google Scholar]
- [22].Oliveira CP, Faintuch J, Rascovski A, Furuya CK, Bastos MoS, Matsuda M, et al. Lipid peroxidation in bariatric candidates with nonalcoholic fatty liver disease (NAFLD) -- preliminary findings. Obes Surg. 2005;15:502–5. doi: 10.1381/0960892053723493. [DOI] [PubMed] [Google Scholar]
- [23].Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quiñones L, et al. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–8. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- [24].Elizondo A, Araya J, Rodrigo R, Signorini C, Sgherri C, Comporti M, et al. Effects of weight loss on liver and erythrocyte polyunsaturated fatty acid pattern and oxidative stress status in obese patients with non-alcoholic fatty liver disease. Biol Res. 2008;41:59–68. [PubMed] [Google Scholar]
- [25].Pettinelli P, Obregón AM, Videla LA. Molecular mechanisms of steatosis in nonalcoholic fatty liver disease. Nutr Hosp. 2011;26:441–50. doi: 10.1590/S0212-16112011000300003. [DOI] [PubMed] [Google Scholar]
- [26].Méndez-Sánchez N, Arrese M, Zamora-Valdés D, Uribe M. Current concepts in the pathogenesis of nonalcoholic fatty liver disease. Liver Int. 2007;27:423–33. doi: 10.1111/j.1478-3231.2007.01483.x. [DOI] [PubMed] [Google Scholar]
- [27].Cortez-Pinto H, de Moura MC, Day CP. Non-alcoholic steatohepatitis: from cell biology to clinical practice. J Hepatol. 2006;44:197–208. doi: 10.1016/j.jhep.2005.09.002. [DOI] [PubMed] [Google Scholar]
- [28].Almeda-Valdés P, Cuevas-Ramos D, Aguilar-Salinas CA. Metabolic syndrome and non-alcoholic fatty liver disease. Ann Hepatol. 2009;8(Suppl 1):S18–24. [PubMed] [Google Scholar]
- [29].Grundy SM, Hansen B, Smith SC, Cleeman JI, Kahn RA, Association AH et al. Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation. 2004;109:551–6. doi: 10.1161/01.CIR.0000112379.88385.67. [DOI] [PubMed] [Google Scholar]
- [30].Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- [31].Brunt EM. Pathology of nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2010;7:195–203. doi: 10.1038/nrgastro.2010.21. [DOI] [PubMed] [Google Scholar]
- [32].Shayman JA, Abe A. Drug induced phospholipidosis: an acquired lysosomal storage disorder. Biochim Biophys Acta. 2013;1831:602–11. doi: 10.1016/j.bbalip.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Dyson JK, Anstee QM, McPherson S. Non-alcoholic fatty liver disease: a practical approach to diagnosis and staging. Frontline Gastroenterol. 2014;5:211–8. doi: 10.1136/flgastro-2013-100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Felker D, Lynn A, Wang S, Johnson DE. Evidence for a potential protective effect of carnitine-pantothenic acid co-treatment on valproic acid-induced hepatotoxicity. Expert Rev Clin Pharmacol. 2014;7:211–8. doi: 10.1586/17512433.2014.871202. [DOI] [PubMed] [Google Scholar]
- [35].Lempérière T. Brief history of the development of valproate in bipolar disorders [Brief history of the development of valproate in bipolar disorders] Encephale. 2001;27:365–72. [PubMed] [Google Scholar]
- [36].Bowden CL. Valproate. Bipolar Disord. 2003;5:189–202. doi: 10.1034/j.1399-5618.2003.00031.x. [DOI] [PubMed] [Google Scholar]
- [37].Calabrese JR, Delucchi GA. Phenomenology of rapid cycling manic depression and its treatment with valproate. J Clin Psychiatry. 1989;50(Suppl):30–4. [PubMed] [Google Scholar]
- [38].Erenberg G, Rothner AD, Henry CE, Cruse RP. Valproic acid in the treatment of intractable absence seizures in children: a single-blind clinical and quantitative EEG study. Am J Dis Child. 1982;136:526–9. doi: 10.1001/archpedi.1982.03970420050011. [DOI] [PubMed] [Google Scholar]
- [39].Coppola G, Auricchio G, Federico R, Carotenuto M, Pascotto A. Lamotrigine versus valproic acid as first-line monotherapy in newly diagnosed typical absence seizures: an open-label, randomized, parallel-group study. Epilepsia. 2004;45:1049–53. doi: 10.1111/j.0013-9580.2004.40903.x. [DOI] [PubMed] [Google Scholar]
- [40].Dean JC, Penry JK. Valproate monotherapy in 30 patients with partial seizures. Epilepsia. 1988;29:140–4. doi: 10.1111/j.1528-1157.1988.tb04409.x. [DOI] [PubMed] [Google Scholar]
- [41].Calleja S, Salas-Puig J, Ribacoba R, Lahoz CH. Evolution of juvenile myoclonic epilepsy treated from the outset with sodium valproate. Seizure. 2001;10:424–7. doi: 10.1053/seiz.2000.0530. [DOI] [PubMed] [Google Scholar]
- [42].Phiel CJ, Zhang F, Huang EY, Guenther MG, Lazar MA, Klein PS. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–41. doi: 10.1074/jbc.M101287200. [DOI] [PubMed] [Google Scholar]
- [43].Nanau RM, Neuman MG. Adverse drug reactions induced by valproic acid. Clin Biochem. 2013;46:1323–38. doi: 10.1016/j.clinbiochem.2013.06.012. [DOI] [PubMed] [Google Scholar]
- [44].Turnbull DM, Bone AJ, Bartlett K, Koundakjian PP, Sherratt HS. The effects of valproate on intermediary metabolism in isolated rat hepatocytes and intact rats. Biochem Pharmacol. 1983;32:1887–92. doi: 10.1016/0006-2952(83)90054-0. [DOI] [PubMed] [Google Scholar]
- [45].Ponchaut S, van Hoof F, Veitch K. In vitro effects of valproate and valproate metabolites on mitochondrial oxidations. Relevance of CoA sequestration to the observed inhibitions. Biochem Pharmacol. 1992;43:2435–42. doi: 10.1016/0006-2952(92)90324-c. [DOI] [PubMed] [Google Scholar]
- [46].Bjorge SM, Baillie TA. Inhibition of medium-chain fatty acid beta-oxidation in vitro by valproic acid and its unsaturated metabolite, 2-n-propyl-4-pentenoic acid. Biochem Biophys Res Commun. 1985;132:245–52. doi: 10.1016/0006-291x(85)91014-9. [DOI] [PubMed] [Google Scholar]
- [47].Lee MH, Kim M, Lee BH, Kim JH, Kang KS, Kim HL, et al. Subchronic effects of valproic acid on gene expression profiles for lipid metabolism in mouse liver. Toxicol Appl Pharmacol. 2008;226:271–84. doi: 10.1016/j.taap.2007.09.014. [DOI] [PubMed] [Google Scholar]
- [48].Knapp AC, Todesco L, Beier K, Terracciano L, Sägesser H, Reichen J, et al. Toxicity of valproic acid in mice with decreased plasma and tissue carnitine stores. J Pharmacol Exp Ther. 2008;324:568–75. doi: 10.1124/jpet.107.131185. [DOI] [PubMed] [Google Scholar]
- [49].Kingsley E, Gray P, Tolman KG, Tweedale R. The toxicity of metabolites of sodium valproate in cultured hepatocytes. J Clin Pharmacol. 1983;23:178–85. doi: 10.1002/j.1552-4604.1983.tb02722.x. [DOI] [PubMed] [Google Scholar]
- [50].Granneman GR, Wang SI, Kesterson JW, Machinist JM. The hepatotoxicity of valproic acid and its metabolites in rats. II. Intermediary and valproic acid metabolism. Hepatology. 1984;4:1153–8. doi: 10.1002/hep.1840040610. [DOI] [PubMed] [Google Scholar]
- [51].Kesterson JW, Granneman GR, Machinist JM. The hepatotoxicity of valproic acid and its metabolites in rats. I. Toxicologic, biochemical and histopathologic studies. Hepatology. 1984;4:1143–52. doi: 10.1002/hep.1840040609. [DOI] [PubMed] [Google Scholar]
- [52].Kassahun K, Farrell K, Abbott F. Identification and characterization of the glutathione and N-acetylcysteine conjugates of (E)-2-propyl-2,4-pentadienoic acid, a toxic metabolite of valproic acid, in rats and humans. Drug Metab Dispos. 1991;19:525–35. [PubMed] [Google Scholar]
- [53].Sadeque AJ, Fisher MB, Korzekwa KR, Gonzalez FJ, Rettie AE. Human CYP2C9 and CYP2A6 mediate formation of the hepatotoxin 4-ene-valproic acid. J Pharmacol Exp Ther. 1997;283:698–703. [PubMed] [Google Scholar]
- [54].Lemasters JJ, Nieminen AL, Qian T, Trost LC, Elmore SP, Nishimura Y, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta. 1998;1366:177–96. doi: 10.1016/s0005-2728(98)00112-1. [DOI] [PubMed] [Google Scholar]
- [55].Aires CC, Ijlst L, Stet F, Prip-Buus C, de Almeida IT, Duran M, et al. Inhibition of hepatic carnitine palmitoyl-transferase I (CPT IA) by valproyl-CoA as a possible mechanism of valproate-induced steatosis. Biochem Pharmacol. 2010;79:792–9. doi: 10.1016/j.bcp.2009.10.011. [DOI] [PubMed] [Google Scholar]
- [56].Van Cott TE, Yehle KS, DeCrane SK, Thorlton JR. Amiodarone-induced pulmonary toxicity: case study with syndrome analysis. Heart Lung. 2013;42:262–6. doi: 10.1016/j.hrtlng.2013.05.004. [DOI] [PubMed] [Google Scholar]
- [57].Stähli BE, Schwab S. Amiodarone-induced skin hyperpigmentation. QJM. 2011;104:723–4. doi: 10.1093/qjmed/hcq131. [DOI] [PubMed] [Google Scholar]
- [58].Preda C, Aprotosoaie AC, Petris A. Costache II. Amiodarone-induced thyroid dysfunction--clinical picture. Study on 215 cases. Rev Med Chir Soc Med Nat Iasi. 2014;118:359–63. [PubMed] [Google Scholar]
- [59].Li JG, Yang TC, Yu DM, Ren TH. Fatal acute liver failure after intravenous amiodarone administration. J Formos Med Assoc. 2013 doi: 10.1016/j.jfma.2013.07.011. [DOI] [PubMed] [Google Scholar]
- [60].Fromenty B, Fisch C, Labbe G, Degott C, Deschamps D, Berson A, et al. Amiodarone inhibits the mitochondrial beta-oxidation of fatty acids and produces microvesicular steatosis of the liver in mice. J Pharmacol Exp Ther. 1990;255:1371–6. [PubMed] [Google Scholar]
- [61].Kumar K, Zimetbaum PJ. Antiarrhythmic drugs 2013: state of the art. Curr Cardiol Rep. 2013;15:410. doi: 10.1007/s11886-013-0410-2. [DOI] [PubMed] [Google Scholar]
- [62].Punnam SR, Goyal SK, Kotaru VP, Pachika AR, Abela GS, Thakur RK. Amiodarone - a ‘broad spectrum’ antiarrhythmic drug. Cardiovasc Hematol Disord Drug Targets. 2010;10:73–81. doi: 10.2174/187152910790780032. [DOI] [PubMed] [Google Scholar]
- [63].McCarthy TC, Pollak PT, Hanniman EA, Sinal CJ. Disruption of hepatic lipid homeostasis in mice after amiodarone treatment is associated with peroxisome proliferator-activated receptor-alpha target gene activation. J Pharmacol Exp Ther. 2004;311:864–73. doi: 10.1124/jpet.104.072785. [DOI] [PubMed] [Google Scholar]
- [64].Deschamps D, DeBeco V, Fisch C, Fromenty B, Guillouzo A, Pessayre D. Inhibition by perhexiline of oxidative phosphorylation and the beta-oxidation of fatty acids: possible role in pseudoalcoholic liver lesions. Hepatology. 1994;19:948–61. [PubMed] [Google Scholar]
- [65].Varbiro G, Toth A, Tapodi A, Veres B, Sumegi B, Gallyas F. Concentration dependent mitochondrial effect of amiodarone. Biochem Pharmacol. 2003;65:1115–28. doi: 10.1016/s0006-2952(02)01660-x. [DOI] [PubMed] [Google Scholar]
- [66].Wetterau JR, Lin MC, Jamil H. Microsomal triglyceride transfer protein. Biochim Biophys Acta. 1997;1345:136–50. doi: 10.1016/s0005-2760(96)00168-3. [DOI] [PubMed] [Google Scholar]
- [67].Felser A, Blum K, Lindinger PW, Bouitbir J, Krähenbühl S. Mechanisms of hepatocellular toxicity associated with dronedarone--a comparison to amiodarone. Toxicol Sci. 2013;131:480–90. doi: 10.1093/toxsci/kfs298. [DOI] [PubMed] [Google Scholar]
- [68].Chopra I, Roberts M. Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol Mol Biol Rev. 2001;65:232–260. doi: 10.1128/MMBR.65.2.232-260.2001. second page, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Thiim M, Friedman LS. Hepatotoxicity of antibiotics and antifungals. Clin Liver Dis. 2003;7:381–99. vi–vii. doi: 10.1016/s1089-3261(03)00021-7. [DOI] [PubMed] [Google Scholar]
- [70].Ford TJ, Dillon JF. Minocycline hepatitis. Eur J Gastroenterol Hepatol. 2008;20:796–9. doi: 10.1097/MEG.0b013e3282f493c5. [DOI] [PubMed] [Google Scholar]
- [71].Yin HQ, Kim M, Kim JH, Kong G, Lee MO, Kang KS, et al. Hepatic gene expression profiling and lipid homeostasis in mice exposed to steatogenic drug, tetracycline. Toxicol Sci. 2006;94:206–16. doi: 10.1093/toxsci/kfl078. [DOI] [PubMed] [Google Scholar]
- [72].Yu HY, Wang BL, Zhao J, Yao XM, Gu Y, Li Y. Protective effect of bicyclol on tetracycline-induced fatty liver in mice. Toxicology. 2009;261:112–8. doi: 10.1016/j.tox.2009.04.058. [DOI] [PubMed] [Google Scholar]
- [73].Journey LJ, Goldstein MN. The effect of terramycin on the fine structure of HeLa cell mitochondria. Cancer Res. 1963;23:551–4. [PubMed] [Google Scholar]
- [74].Du Buy HG, Showacre JL. Selective localization of tetracycline in mitochondria of living cells. Science. 1961;133:196–7. doi: 10.1126/science.133.3447.196. [DOI] [PubMed] [Google Scholar]
- [75].Schultz JC, Adamson JS, Workman WW, Norman TD. Fatal liver disease after intravenous administration of tetracycline in high dosage. N Engl J Med. 1963;269:999–1004. doi: 10.1056/NEJM196311072691903. [DOI] [PubMed] [Google Scholar]
- [76].Peters RL, Edmondson HA, Mikkelsen WP, Tatter D. Tetracycline-induced fatty liver in nonpregnant patients. A report of six cases. Am J Surg. 1967;113:622–32. doi: 10.1016/0002-9610(67)90308-x. [DOI] [PubMed] [Google Scholar]
- [77].Griffin MO, Fricovsky E, Ceballos G, Villarreal F. Tetracyclines: a pleitropic family of compounds with promising therapeutic properties. Review of the literature. Am J Physiol Cell Physiol. 2010;299:C539–48. doi: 10.1152/ajpcell.00047.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Chang J, Powles TJ, Ashley SE, Gregory RK, Tidy VA, Treleaven JG, et al. The effect of tamoxifen and hormone replacement therapy on serum cholesterol, bone mineral density and coagulation factors in healthy postmenopausal women participating in a randomised, controlled tamoxifen prevention study. Ann Oncol. 1996;7:671–5. doi: 10.1093/oxfordjournals.annonc.a010715. [DOI] [PubMed] [Google Scholar]
- [79].Bradbury BD, Lash TL, Kaye JA, Jick SS. Tamoxifen-treated breast carcinoma patients and the risk of acute myocardial infarction and newly-diagnosed angina. Cancer. 2005;103:1114–21. doi: 10.1002/cncr.20900. [DOI] [PubMed] [Google Scholar]
- [80].Shiau AK, Barstad D, Loria PM, Cheng L, Kushner PJ, Agard DA, et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell. 1998;95:927–37. doi: 10.1016/s0092-8674(00)81717-1. [DOI] [PubMed] [Google Scholar]
- [81].(EBCTCG) EBCTCG Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687–717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- [82].Nishino M, Hayakawa K, Nakamura Y, Morimoto T, Mukaihara S. Effects of tamoxifen on hepatic fat content and the development of hepatic steatosis in patients with breast cancer: high frequency of involvement and rapid reversal after completion of tamoxifen therapy. AJR Am J Roentgenol. 2003;180:129–34. doi: 10.2214/ajr.180.1.1800129. [DOI] [PubMed] [Google Scholar]
- [83].Murata Y, Ogawa Y, Saibara T, Nishioka A, Fujiwara Y, Fukumoto M, et al. Unrecognized hepatic steatosis and non-alcoholic steatohepatitis in adjuvant tamoxifen for breast cancer patients. Oncol Rep. 2000;7:1299–304. doi: 10.3892/or.7.6.1299. [DOI] [PubMed] [Google Scholar]
- [84].Oien KA, Moffat D, Curry GW, Dickson J, Habeshaw T, Mills PR, et al. Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet. 1999;353:36–7. doi: 10.1016/S0140-6736(05)74872-8. [DOI] [PubMed] [Google Scholar]
- [85].Lee MH, Kim JW, Kim JH, Kang KS, Kong G, Lee MO. Gene expression profiling of murine hepatic steatosis induced by tamoxifen. Toxicol Lett. 2010;199:416–24. doi: 10.1016/j.toxlet.2010.10.008. [DOI] [PubMed] [Google Scholar]
- [86].Gudbrandsen OA, Rost TH, Berge RK. Causes and prevention of tamoxifen-induced accumulation of triacylglycerol in rat liver. J Lipid Res. 2006;47:2223–32. doi: 10.1194/jlr.M600148-JLR200. [DOI] [PubMed] [Google Scholar]
- [87].Cardoso CM, Custódio JB, Almeida LM, Moreno AJ. Mechanisms of the deleterious effects of tamoxifen on mitochondrial respiration rate and phosphorylation efficiency. Toxicol Appl Pharmacol. 2001;176:145–52. doi: 10.1006/taap.2001.9265. [DOI] [PubMed] [Google Scholar]
- [88].Tuquet C, Dupont J, Mesneau A, Roussaux J. Effects of tamoxifen on the electron transport chain of isolated rat liver mitochondria. Cell Biol Toxicol. 2000;16:207–19. doi: 10.1023/a:1007695308257. [DOI] [PubMed] [Google Scholar]
- [89].Starko KM, Ray CG, Dominguez LB, Stromberg WL, Woodall DF. Reye’s syndrome and salicylate use. Pediatrics. 1980;66:859–64. [PubMed] [Google Scholar]
- [90].Reye RD, Morgan G, Baral J. Encephalopathy and fatty degeneration of the viscera. A disease entity in childhood. Lancet. 1963;2:749–52. doi: 10.1016/s0140-6736(63)90554-3. [DOI] [PubMed] [Google Scholar]
- [91].Segalman TY, Lee CP. Reye’s syndrome: plasma-induced alterations in mitochondrial structure and function. Arch Biochem Biophys. 1982;214:522–30. doi: 10.1016/0003-9861(82)90056-x. [DOI] [PubMed] [Google Scholar]
- [92].You K. Salicylate and mitochondrial injury in Reye’s syndrome. Science. 1983;221:163–5. doi: 10.1126/science.6857275. [DOI] [PubMed] [Google Scholar]
- [93].Martens ME, Chang CH, Lee CP. Reye’s syndrome: mitochondrial swelling and Ca2+ release induced by Reye’s plasma, allantoin, and salicylate. Arch Biochem Biophys. 1986;244:773–86. doi: 10.1016/0003-9861(86)90646-6. [DOI] [PubMed] [Google Scholar]
- [94].Browne GS, Nelson C, Nguyen T, Ellis BA, Day RO, Williams KM. Stereoselective and substrate-dependent inhibition of hepatic mitochondria beta-oxidation and oxidative phosphorylation by the non-steroidal anti-inflammatory drugs ibuprofen, flurbiprofen, and ketorolac. Biochem Pharmacol. 1999;57:837–44. doi: 10.1016/s0006-2952(98)00342-6. [DOI] [PubMed] [Google Scholar]
- [95].Freneaux E, Fromenty B, Berson A, Labbe G, Degott C, Letteron P, et al. Stereoselective and nonstereoselective effects of ibuprofen enantiomers on mitochondrial beta-oxidation of fatty acids. J Pharmacol Exp Ther. 1990;255:529–35. [PubMed] [Google Scholar]
- [96].Lettéron P, Sutton A, Mansouri A, Fromenty B, Pessayre D. Inhibition of microsomal triglyceride transfer protein: another mechanism for drug-induced steatosis in mice. Hepatology. 2003;38:133–40. doi: 10.1053/jhep.2003.50309. [DOI] [PubMed] [Google Scholar]
- [97].Berson A, Cazanave S, Descatoire V, Tinel M, Grodet A, Wolf C, et al. The anti-inflammatory drug, nimesulide (4-nitro-2-phenoxymethane-sulfoanilide), uncouples mitochondria and induces mitochondrial permeability transition in human hepatoma cells: protection by albumin. J Pharmacol Exp Ther. 2006;318:444–54. doi: 10.1124/jpet.106.104125. [DOI] [PubMed] [Google Scholar]
- [98].Panatto JP, Jeremias IC, Ferreira GK, Ramos AC, Rochi N, Gonçalves CL, et al. Inhibition of mitochondrial respiratory chain in the brain of rats after hepatic failure induced by acetaminophen. Mol Cell Biochem. 2011;350:149–54. doi: 10.1007/s11010-010-0689-x. [DOI] [PubMed] [Google Scholar]
- [99].McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–83. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–6. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- [101].Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015 doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Swaminath A, Taunk R, Lawlor G. Use of methotrexate in inflammatory bowel disease in 2014: A User’s Guide. World J Gastrointest Pharmacol Ther. 2014;5:113–21. doi: 10.4292/wjgpt.v5.i3.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].König J, Seithel A, Gradhand U, Fromm MF. Pharmacogenomics of human OATP transporters. Naunyn Schmiedebergs Arch Pharmacol. 2006;372:432–43. doi: 10.1007/s00210-006-0040-y. [DOI] [PubMed] [Google Scholar]
- [104].Kolli V, Natarajan K, Isaac B, Selvakumar D, Abraham P. Mitochondrial dysfunction and respiratory chain defects in a rodent model of methotrexate-induced enteritis. Hum Exp Toxicol. 2014;33:1051–65. doi: 10.1177/0960327113515503. [DOI] [PubMed] [Google Scholar]
- [105].Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol. 2009;62:481–92. doi: 10.1136/jcp.2008.058248. [DOI] [PubMed] [Google Scholar]
- [106].Vauthey JN, Pawlik TM, Ribero D, Wu TT, Zorzi D, Hoff PM, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–72. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- [107].Le Dinh T, Freneaux E, Labbe G, Letteron P, Degott C, Geneve J, et al. Amineptine, a tricyclic antidepressant, inhibits the mitochondrial oxidation of fatty acids and produces microvesicular steatosis of the liver in mice. J Pharmacol Exp Ther. 1988;247:745–50. [PubMed] [Google Scholar]
- [108].Fromenty B, Freneaux E, Labbe G, Deschamps D, Larrey D, Letteron P, et al. Tianeptine, a new tricyclic antidepressant metabolized by beta-oxidation of its heptanoic side chain, inhibits the mitochondrial oxidation of medium and short chain fatty acids in mice. Biochem Pharmacol. 1989;38:3743–51. doi: 10.1016/0006-2952(89)90580-7. [DOI] [PubMed] [Google Scholar]
- [109].Igoudjil A, Massart J, Begriche K, Descatoire V, Robin MA, Fromenty B. High concentrations of stavudine impair fatty acid oxidation without depleting mitochondrial DNA in cultured rat hepatocytes. Toxicol In Vitro. 2008;22:887–98. doi: 10.1016/j.tiv.2008.01.011. [DOI] [PubMed] [Google Scholar]
- [110].Walker UA, Bäuerle J, Laguno M, Murillas J, Mauss S, Schmutz G, et al. Depletion of mitochondrial DNA in liver under antiretroviral therapy with didanosine, stavudine, or zalcitabine. Hepatology. 2004;39:311–7. doi: 10.1002/hep.20074. [DOI] [PubMed] [Google Scholar]
- [111].Kennedy JA, Unger SA, Horowitz JD. Inhibition of carnitine palmitoyltransferase-1 in rat heart and liver by perhexiline and amiodarone. Biochem Pharmacol. 1996;52:273–80. doi: 10.1016/0006-2952(96)00204-3. [DOI] [PubMed] [Google Scholar]
- [112].Lewis D, Wainwright HC, Kew MC, Zwi S, Isaacson C. Liver damage associated with perhexiline maleate. Gut. 1979;20:186–9. doi: 10.1136/gut.20.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jazcilevich S, Villa-Treviño S. Induction of fatty liver in the rat after cycloheximide administration. Lab Invest. 1970;23:590–4. [PubMed] [Google Scholar]
- [114].García-Sáinz JA, Hernández-Muñoz R, Santamaría A, de Sánchez VC. Mechanism of the fatty liver induced by cycloheximide and its reversibility by adenosine. Biochem Pharmacol. 1979;28:1409–13. doi: 10.1016/0006-2952(79)90444-1. [DOI] [PubMed] [Google Scholar]
- [115].Bar-On H, Stein O, Stein Y. Mulitiple effects of cycloheximide on the metabolism of triglycerides in the liver of male and female rats. Biochim Biophys Acta. 1972;270:444–52. doi: 10.1016/0005-2760(72)90109-9. [DOI] [PubMed] [Google Scholar]
- [116].Murakami M, Bessho K, Mushiake S, Kondou H, Miyoshi Y, Ozono K. Major role of apolipoprotein B in cycloheximide-induced acute hepatic steatosis in mice. Hepatol Res. 2011;41:446–54. doi: 10.1111/j.1872-034X.2011.00791.x. [DOI] [PubMed] [Google Scholar]
- [117].Mach T, Janik A. The effect of prolonged administration of disulfiram alone or in combination with ethanol on some indices of lipid metabolism in the blood serum and hepatic tissue of the rat. Pol J Pharmacol Pharm. 1980;32:327–32. [PubMed] [Google Scholar]
- [118].Balakirev MY, Zimmer G. Mitochondrial injury by disulfiram: two different mechanisms of the mitochondrial permeability transition. Chem Biol Interact. 2001;138:299–311. doi: 10.1016/s0009-2797(01)00283-6. [DOI] [PubMed] [Google Scholar]
- [119].Kuroda MA, Cuéllar A. Deleterious effects of disulfiram on the respiratory electron transport system of liver mitochondria. Int J Biochem. 1993;25:87–91. doi: 10.1016/0020-711x(93)90493-x. [DOI] [PubMed] [Google Scholar]
- [120].Horn DM, Neeb LA, Colacino JM, Richardson FC. Fialuridine is phosphorylated and inhibits DNA synthesis in isolated rat hepatic mitochondria. Antiviral Res. 1997;34:71–4. doi: 10.1016/s0166-3542(96)01027-3. [DOI] [PubMed] [Google Scholar]
- [121].Berson A, Descatoire V, Sutton A, Fau D, Maulny B, Vadrot N, et al. Toxicity of alpidem, a peripheral benzodiazepine receptor ligand, but not zolpidem, in rat hepatocytes: role of mitochondrial permeability transition and metabolic activation. J Pharmacol Exp Ther. 2001;299:793–800. [PubMed] [Google Scholar]
- [122].Berson A, Schmets L, Fisch C, Fau D, Wolf C, Fromenty B, et al. Inhibition by nilutamide of the mitochondrial respiratory chain and ATP formation. Possible contribution to the adverse effects of this antiandrogen. J Pharmacol Exp Ther. 1994;270:167–76. [PubMed] [Google Scholar]
- [123].Rachek LI, Yuzefovych LV, Ledoux SP, Julie NL, Wilson GL. Troglitazone, but not rosiglitazone, damages mitochondrial DNA and induces mitochondrial dysfunction and cell death in human hepatocytes. Toxicol Appl Pharmacol. 2009;240:348–54. doi: 10.1016/j.taap.2009.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Okuda T, Norioka M, Shitara Y, Horie T. Multiple mechanisms underlying troglitazone-induced mitochondrial permeability transition. Toxicol Appl Pharmacol. 2010;248:242–8. doi: 10.1016/j.taap.2010.08.007. [DOI] [PubMed] [Google Scholar]
- [125].Melo T, Videira RA, André S, Maciel E, Francisco CS, Oliveira-Campos AM, et al. Tacrine and its analogues impair mitochondrial function and bioenergetics: a lipidomic analysis in rat brain. J Neurochem. 2012;120:998–1013. doi: 10.1111/j.1471-4159.2011.07636.x. [DOI] [PubMed] [Google Scholar]
- [126].Mansouri A, Haouzi D, Descatoire V, Demeilliers C, Sutton A, Vadrot N, et al. Tacrine inhibits topoisomerases and DNA synthesis to cause mitochondrial DNA depletion and apoptosis in mouse liver. Hepatology. 2003;38:715–25. doi: 10.1053/jhep.2003.50353. [DOI] [PubMed] [Google Scholar]
- [127].Tanaka S, Shimonaka H, Tanahashi T, Uematsu H, Yamamoto M, Kawai K, et al. [The effect of buprenorphine on oxidative phosphorylation in isolated rat liver mitochondria] Masui. 1985;34:1456–62. [PubMed] [Google Scholar]
- [128].Feo C, Garçon E. Study of steatosis induced in the rat by orotic acid. Biomedicine. 1973;19:61–4. [PubMed] [Google Scholar]
- [129].Zhang WV, Ramzan I, Murray M. Impaired microsomal oxidation of the atypical antipsychotic agent clozapine in hepatic steatosis. J Pharmacol Exp Ther. 2007;322:770–7. doi: 10.1124/jpet.107.124024. [DOI] [PubMed] [Google Scholar]
- [130].Hammes TO, Pedroso GL, Hartmann CR, Escobar TD, Fracasso LB, da Rosa DP, et al. The effect of taurine on hepatic steatosis induced by thioacetamide in zebrafish (Danio rerio) Dig Dis Sci. 2012;57:675–82. doi: 10.1007/s10620-011-1931-4. [DOI] [PubMed] [Google Scholar]
- [131].Takahashi Y, Soejima Y, Fukusato T. Animal models of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2012;18:2300–8. doi: 10.3748/wjg.v18.i19.2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Kanuri G, Bergheim I. In Vitro and in Vivo Models of Non-Alcoholic Fatty Liver Disease (NAFLD) Int J Mol Sci. 2013;14:11963–80. doi: 10.3390/ijms140611963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Nanji AA. Role of different dietary fatty acids in the pathogenesis of experimental alcoholic liver disease. Alcohol. 2004;34:21–5. doi: 10.1016/j.alcohol.2004.08.005. [DOI] [PubMed] [Google Scholar]
- [134].van den Berg SA, Guigas B, Bijland S, Ouwens M, Voshol PJ, Frants RR, et al. High levels of dietary stearate promote adiposity and deteriorate hepatic insulin sensitivity. Nutr Metab (Lond) 2010;7:24. doi: 10.1186/1743-7075-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–75. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Larter CZ, Yeh MM, Williams J, Bell-Anderson KS, Farrell GC. MCD-induced steatohepatitis is associated with hepatic adiponectin resistance and adipogenic transformation of hepatocytes. J Hepatol. 2008;49:407–16. doi: 10.1016/j.jhep.2008.03.026. [DOI] [PubMed] [Google Scholar]
- [138].Rinella ME, Elias MS, Smolak RR, Fu T, Borensztajn J, Green RM. Mechanisms of hepatic steatosis in mice fed a lipogenic methionine choline-deficient diet. J Lipid Res. 2008;49:1068–76. doi: 10.1194/jlr.M800042-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Fan JG, Qiao L. Commonly used animal models of non-alcoholic steatohepatitis. Hepatobiliary Pancreat Dis Int. 2009;8:233–40. [PubMed] [Google Scholar]
- [140].De Minicis S, Agostinelli L, Rychlicki C, Sorice GP, Saccomanno S, Candelaresi C, et al. HCC development is associated to peripheral insulin resistance in a mouse model of NASH. PLoS One. 2014;9:e97136. doi: 10.1371/journal.pone.0097136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Pogribny IP, Tryndyak VP, Muskhelishvili L, Rusyn I, Ross SA. Methyl deficiency, alterations in global histone modifications, and carcinogenesis. J Nutr. 2007;137:216S–22S. doi: 10.1093/jn/137.1.216S. [DOI] [PubMed] [Google Scholar]
- [142].Pogribny IP, Tryndyak VP, Bagnyukova TV, Melnyk S, Montgomery B, Ross SA, et al. Hepatic epigenetic phenotype predetermines individual susceptibility to hepatic steatosis in mice fed a lipogenic methyl-deficient diet. J Hepatol. 2009;51:176–86. doi: 10.1016/j.jhep.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Ackerman Z, Oron-Herman M, Grozovski M, Rosenthal T, Pappo O, Link G, et al. Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension. 2005;45:1012–8. doi: 10.1161/01.HYP.0000164570.20420.67. [DOI] [PubMed] [Google Scholar]
- [144].Spruss A, Kanuri G, Wagnerberger S, Haub S, Bischoff SC, Bergheim I. Toll-like receptor 4 is involved in the development of fructose-induced hepatic steatosis in mice. Hepatology. 2009;50:1094–04. doi: 10.1002/hep.23122. [DOI] [PubMed] [Google Scholar]
- [145].Kawasaki T, Igarashi K, Koeda T, Sugimoto K, Nakagawa K, Hayashi S, et al. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J Nutr. 2009;139:2067–71. doi: 10.3945/jn.109.105858. [DOI] [PubMed] [Google Scholar]
- [146].Schultz A, Neil D, Aguila MB, Mandarim-de-Lacerda CA. Hepatic adverse effects of fructose consumption independent of overweight/obesity. Int J Mol Sci. 2013;14:21873–86. doi: 10.3390/ijms141121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Charlton M, Krishnan A, Viker K, Sanderson S, Cazanave S, McConico A, et al. Fast food diet mouse: novel small animal model of NASH with ballooning, progressive fibrosis, and high physiological fidelity to the human condition. Am J Physiol Gastrointest Liver Physiol. 2011;301:G825–34. doi: 10.1152/ajpgi.00145.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Lopez S, Bermudez B, Montserrat-de la Paz S, Jaramillo S, Varela LM, Ortega-Gomez A, et al. Membrane composition and dynamics: a target of bioactive virgin olive oil constituents. Biochim Biophys Acta. 2014;1838:1638–56. doi: 10.1016/j.bbamem.2014.01.007. [DOI] [PubMed] [Google Scholar]
- [149].Baylin A, Deka R, Tuitele J, Viali S, Weeks DE, McGarvey ST. INSIG2 variants, dietary patterns and metabolic risk in Samoa. Eur J Clin Nutr. 2013;67:101–7. doi: 10.1038/ejcn.2012.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kotronen A, Yki-Järvinen H, Sevastianova K, Bergholm R, Hakkarainen A, Pietiläinen KH, et al. Comparison of the relative contributions of intra-abdominal and liver fat to components of the metabolic syndrome. Obesity (Silver Spring) 2011;19:23–8. doi: 10.1038/oby.2010.137. [DOI] [PubMed] [Google Scholar]
- [151].Andersson A, Nälsén C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr. 2002;76:1222–9. doi: 10.1093/ajcn/76.6.1222. [DOI] [PubMed] [Google Scholar]
- [152].Rocquelin G, Guenot L, Astorg PO, David M. Phospholipid content and fatty acid composition of human heart. Lipids. 1989;24:775–80. doi: 10.1007/BF02544583. [DOI] [PubMed] [Google Scholar]
- [153].Bahrami G, Masoumi M, Rahimi Z. Co-existence of fatty acids changes in aorta artery and adipose tissue; comparison between CAD and non CAD patients. J Thromb Thrombolysis. 2009;27:185–90. doi: 10.1007/s11239-008-0198-x. [DOI] [PubMed] [Google Scholar]
- [154].Fraser T, Tayler H, Love S. Fatty acid composition of frontal, temporal and parietal neocortex in the normal human brain and in Alzheimer’s disease. Neurochem Res. 2010;35:503–13. doi: 10.1007/s11064-009-0087-5. [DOI] [PubMed] [Google Scholar]
- [155].Guest J, Garg M, Bilgin A, Grant R. Relationship between central and peripheral fatty acids in humans. Lipids Health Dis. 2013;12:79. doi: 10.1186/1476-511X-12-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Araya J, Rodrigo R, Videla LA, Thielemann L, Orellana M, Pettinelli P, et al. Increase in long-chain polyunsaturated fatty acid n - 6/n - 3 ratio in relation to hepatic steatosis in patients with non-alcoholic fatty liver disease. Clin Sci (Lond) 2004;106:635–43. doi: 10.1042/CS20030326. [DOI] [PubMed] [Google Scholar]
- [157].Martin G, Nemoto M, Gelman L, Geffroy S, Najib J, Fruchart JC, et al. The human fatty acid transport protein-1 (SLC27A1; FATP-1) cDNA and gene: organization, chromosomal localization, and expression. Genomics. 2000;66:296–304. doi: 10.1006/geno.2000.6191. [DOI] [PubMed] [Google Scholar]
- [158].Ge F, Zhou S, Hu C, Lobdell H, Berk PD. Insulin- and leptin-regulated fatty acid uptake plays a key causal role in hepatic steatosis in mice with intact leptin signaling but not in ob/ob or db/db mice. Am J Physiol Gastrointest Liver Physiol. 2010;299:G855–66. doi: 10.1152/ajpgi.00434.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Zhou SL, Stump D, Sorrentino D, Potter BJ, Berk PD. Adipocyte differentiation of 3T3-L1 cells involves augmented expression of a 43-kDa plasma membrane fatty acid-binding protein. J Biol Chem. 1992;267:14456–61. [PubMed] [Google Scholar]
- [160].Zhou SL, Stump D, Kiang CL, Isola LM, Berk PD. Mitochondrial aspartate aminotransferase expressed on the surface of 3T3-L1 adipocytes mediates saturable fatty acid uptake. Proc Soc Exp Biol Med. 1995;208:263–70. doi: 10.3181/00379727-208-43854. [DOI] [PubMed] [Google Scholar]
- [161].Trigatti BL, Anderson RG, Gerber GE. Identification of caveolin-1 as a fatty acid binding protein. Biochem Biophys Res Commun. 1999;255:34–9. doi: 10.1006/bbrc.1998.0123. [DOI] [PubMed] [Google Scholar]
- [162].Pessayre D, Mansouri A, Berson A, Fromenty B. Mitochondrial involvement in drug-induced liver injury. Handb Exp Pharmacol. 2010:311–65. doi: 10.1007/978-3-642-00663-0_11. [DOI] [PubMed] [Google Scholar]
- [163].De Gottardi A, Vinciguerra M, Sgroi A, Moukil M, Ravier-Dall’Antonia F, Pazienza V, et al. Microarray analyses and molecular profiling of steatosis induction in immortalized human hepatocytes. Lab Invest. 2007;87:792–806. doi: 10.1038/labinvest.3700590. [DOI] [PubMed] [Google Scholar]
- [164].Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–57. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- [165].Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–51. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Bradbury MW. Lipid metabolism and liver inflammation. I. Hepatic fatty acid uptake: possible role in steatosis. Am J Physiol Gastrointest Liver Physiol. 2006;290:G194–198. doi: 10.1152/ajpgi.00413.2005. [DOI] [PubMed] [Google Scholar]
- [167].Niklas J, Bonin A, Mangin S, Bucher J, Kopacz S, Matz-Soja M, et al. Central energy metabolism remains robust in acute steatotic hepatocytes challenged by a high free fatty acid load. BMB Rep. 2012;45:396–401. doi: 10.5483/bmbrep.2012.45.7.070. [DOI] [PubMed] [Google Scholar]
- [168].Cui W, Chen SL, Hu KQ. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am J Transl Res. 2010;2:95–104. [PMC free article] [PubMed] [Google Scholar]
- [169].Zhang D, Xie L, Jia G, Cai S, Ji B, Liu Y, et al. Comparative study on antioxidant capacity of flavonoids and their inhibitory effects on oleic acid-induced hepatic steatosis in vitro. Eur J Med Chem. 2011;46:4548–58. doi: 10.1016/j.ejmech.2011.07.031. [DOI] [PubMed] [Google Scholar]
- [170].Ziamajidi N, Khaghani S, Hassanzadeh G, Vardasbi S, Ahmadian S, Nowrouzi A, et al. Amelioration by chicory seed extract of diabetes- and oleic acid-induced non-alcoholic fatty liver disease (NAFLD)/non-alcoholic steatohepatitis (NASH) via modulation of PPARα and SREBP-1. Food Chem Toxicol. 2013;58:198–209. doi: 10.1016/j.fct.2013.04.018. [DOI] [PubMed] [Google Scholar]
- [171].Kirsch R, Clarkson V, Shephard EG, Marais DA, Jaffer MA, Woodburne VE, et al. Rodent nutritional model of non-alcoholic steatohepatitis: species, strain and sex difference studies. J Gastroenterol Hepatol. 2003;18:1272–82. doi: 10.1046/j.1440-1746.2003.03198.x. [DOI] [PubMed] [Google Scholar]
- [172].Stöppeler S, Palmes D, Fehr M, Hölzen JP, Zibert A, Siaj R, et al. Gender and strain-specific differences in the development of steatosis in rats. Lab Anim. 2013;47:43–52. doi: 10.1177/0023677212473717. [DOI] [PubMed] [Google Scholar]
- [173].Letteron P, Fromenty B, Terris B, Degott C, Pessayre D. Acute and chronic hepatic steatosis lead to in vivo lipid peroxidation in mice. J Hepatol. 1996;24:200–8. doi: 10.1016/s0168-8278(96)80030-4. [DOI] [PubMed] [Google Scholar]
- [174].Tong V, Teng XW, Chang TK, Abbott FS. Valproic acid I: time course of lipid peroxidation biomarkers, liver toxicity, and valproic acid metabolite levels in rats. Toxicol Sci. 2005;86:427–35. doi: 10.1093/toxsci/kfi184. [DOI] [PubMed] [Google Scholar]
- [175].Abdel-Dayem MA, Elmarakby AA, Abdel-Aziz AA, Pye C, Said SA, El-Mowafy AM. Valproate-induced liver injury: modulation by the omega-3 fatty acid DHA proposes a novel anticonvulsant regimen. Drugs R D. 2014;14:85–94. doi: 10.1007/s40268-014-0042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].Lee MH, Hong I, Kim M, Lee BH, Kim JH, Kang KS, et al. Gene expression profiles of murine fatty liver induced by the administration of valproic acid. Toxicol Appl Pharmacol. 2007;220:45–59. doi: 10.1016/j.taap.2006.12.016. [DOI] [PubMed] [Google Scholar]
- [177].Natarajan SK, Eapen CE, Pullimood AB, Balasubramanian KA. Oxidative stress in experimental liver microvesicular steatosis: role of mitochondria and peroxisomes. J Gastroenterol Hepatol. 2006;21:1240–9. doi: 10.1111/j.1440-1746.2006.04313.x. [DOI] [PubMed] [Google Scholar]
- [178].Costa ML, Lima-Júnior RC, Aragão KS, Medeiros RP, Marques-Neto RD, de Sá Grassi L, et al. Chemotherapy-associated steatohepatitis induced by irinotecan: a novel animal model. Cancer Chemother Pharmacol. 2014;74:711–20. doi: 10.1007/s00280-014-2434-8. [DOI] [PubMed] [Google Scholar]
- [179].Nishio S, Gibert Y, Bernard L, Brunet F, Triqueneaux G, Laudet V. Adiponectin and adiponectin receptor genes are coexpressed during zebrafish embryogenesis and regulated by food deprivation. Dev Dyn. 2008;237:1682–90. doi: 10.1002/dvdy.21559. [DOI] [PubMed] [Google Scholar]
- [180].Ibabe A, Grabenbauer M, Baumgart E, Fahimi HD, Cajaraville MP. Expression of peroxisome proliferator-activated receptors in zebrafish (Danio rerio) Histochem Cell Biol. 2002;118:231–9. doi: 10.1007/s00418-002-0434-y. [DOI] [PubMed] [Google Scholar]
- [181].Amali AA, Rekha RD, Lin CJ, Wang WL, Gong HY, Her GM, et al. Thioacetamide induced liver damage in zebrafish embryo as a disease model for steatohepatitis. J Biomed Sci. 2006;13:225–32. doi: 10.1007/s11373-005-9055-5. [DOI] [PubMed] [Google Scholar]
- [182].Howarth DL, Yin C, Yeh K, Sadler KC. Defining hepatic dysfunction parameters in two models of fatty liver disease in zebrafish larvae. Zebrafish. 2013;10:199–210. doi: 10.1089/zeb.2012.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Tainaka T, Shimada Y, Kuroyanagi J, Zang L, Oka T, Nishimura Y, et al. Transcriptome analysis of anti-fatty liver action by Campari tomato using a zebrafish diet-induced obesity model. Nutr Metab (Lond) 2011;8:88. doi: 10.1186/1743-7075-8-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Hiramitsu M, Shimada Y, Kuroyanagi J, Inoue T, Katagiri T, Zang L, et al. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci Rep. 2014;4:3708. doi: 10.1038/srep03708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Sapp V, Gaffney L, EauClaire SF, Matthews RP. Fructose leads to hepatic steatosis in zebrafish that is reversed by mechanistic target of rapamycin (mTOR) inhibition. Hepatology. 2014;60:1581–92. doi: 10.1002/hep.27284. [DOI] [PubMed] [Google Scholar]
- [186].Lindström P. The physiology of obese-hyperglycemic mice [ob/ob mice] ScientificWorldJournal. 2007;7:666–85. doi: 10.1100/tsw.2007.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci U S A. 1997;94:2557–62. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Wortham M, He L, Gyamfi M, Copple BL, Wan YJ. The transition from fatty liver to NASH associates with SAMe depletion in db/db mice fed a methionine choline-deficient diet. Dig Dis Sci. 2008;53:2761–74. doi: 10.1007/s10620-007-0193-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Ross SR, Graves RA, Spiegelman BM. Targeted expression of a toxin gene to adipose tissue: transgenic mice resistant to obesity. Genes Dev. 1993;7:1318–24. doi: 10.1101/gad.7.7b.1318. [DOI] [PubMed] [Google Scholar]
- [190].Shimano H, Horton JD, Hammer RE, Shimomura I, Brown MS, Goldstein JL. Overproduction of cholesterol and fatty acids causes massive liver enlargement in transgenic mice expressing truncated SREBP-1a. J Clin Invest. 1996;98:1575–84. doi: 10.1172/JCI118951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Shimano H, Yahagi N, Amemiya-Kudo M, Hasty AH, Osuga J, Tamura Y, et al. Sterol regulatory element-binding protein-1 as a key transcription factor for nutritional induction of lipogenic enzyme genes. J Biol Chem. 1999;274:35832–9. doi: 10.1074/jbc.274.50.35832. [DOI] [PubMed] [Google Scholar]
- [192].Shimomura I, Hammer RE, Ikemoto S, Brown MS, Goldstein JL. Leptin reverses insulin resistance and diabetes mellitus in mice with congenital lipodystrophy. Nature. 1999;401:73–6. doi: 10.1038/43448. [DOI] [PubMed] [Google Scholar]
- [193].Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999;274:19055–62. doi: 10.1074/jbc.274.27.19055. [DOI] [PubMed] [Google Scholar]
- [194].Simon Y, Kessler SM, Gemperlein K, Bohle RM, Müller R, Haybaeck J, et al. Elevated free cholesterol in a p62 overexpression model of non-alcoholic steatohepatitis. World J Gastroenterol. 2014;20:17839–50. doi: 10.3748/wjg.v20.i47.17839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Lu M, Nakamura RM, Dent ED, Zhang JY, Nielsen FC, Christiansen J, et al. Aberrant expression of fetal RNA-binding protein p62 in liver cancer and liver cirrhosis. Am J Pathol. 2001;159:945–53. doi: 10.1016/S0002-9440(10)61770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Simon Y, Kessler SM, Bohle RM, Haybaeck J, Kiemer AK. The insulin-like growth factor 2 (IGF2) mRNA-binding protein p62/IGF2BP2-2 as a promoter of NAFLD and HCC? Gut. 2014;63:861–3. doi: 10.1136/gutjnl-2013-305736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Tybl E, Shi FD, Kessler SM, Tierling S, Walter J, Bohle RM, et al. Overexpression of the IGF2-mRNA binding protein p62 in transgenic mice induces a steatotic phenotype. J Hepatol. 2011;54:994–1001. doi: 10.1016/j.jhep.2010.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Dongiovanni P, Donati B, Fares R, Lombardi R, Mancina RM, Romeo S, et al. PNPLA3 I148M polymorphism and progressive liver disease. World J Gastroenterol. 2013;19:6969–78. doi: 10.3748/wjg.v19.i41.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Smagris E, BasuRay S, Li J, Huang Y, Lai KM, Gromada J, et al. Pnpla3I148M knockin mice accumulate PNPLA3 on lipid droplets and develop hepatic steatosis. Hepatology. 2015;61:108–18. doi: 10.1002/hep.27242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Garcia Caraballo SC, Comhair TM, Dejong CH, Lamers WH, Köhler SE. A high-protein diet is anti-steatotic and has no pro-inflammatory side effects in dyslipidaemic APOE2 knock-in mice. Br J Nutr. 2014;112:1251–65. doi: 10.1017/S0007114514001986. [DOI] [PubMed] [Google Scholar]
- [201].Nussbaum JM, Liu LJ, Hasan SA, Schaub M, McClendon A, Stainier DY, et al. Homeostatic generation of reactive oxygen species protects the zebrafish liver from steatosis. Hepatology. 2013;58:1326–38. doi: 10.1002/hep.26551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Pai WY, Hsu CC, Lai CY, Chang TZ, Tsai YL, Her GM. Cannabinoid receptor 1 promotes hepatic lipid accumulation and lipotoxicity through the induction of SREBP-1c expression in zebrafish. Transgenic Res. 2013;22:823–38. doi: 10.1007/s11248-012-9685-0. [DOI] [PubMed] [Google Scholar]
- [203].Her GM, Pai WY, Lai CY, Hsieh YW, Pang HW. Ubiquitous transcription factor YY1 promotes zebrafish liver steatosis and lipotoxicity by inhibiting CHOP-10 expression. Biochim Biophys Acta. 2013;1831:1037–51. doi: 10.1016/j.bbalip.2013.02.002. [DOI] [PubMed] [Google Scholar]
- [204].Ji C, Kaplowitz N. ER stress: can the liver cope? J Hepatol. 2006;45:321–33. doi: 10.1016/j.jhep.2006.06.004. [DOI] [PubMed] [Google Scholar]
- [205].Thakur PC, Stuckenholz C, Rivera MR, Davison JM, Yao JK, Amsterdam A, et al. Lack of de novo phosphatidylinositol synthesis leads to endoplasmic reticulum stress and hepatic steatosis in cdipt-deficient zebrafish. Hepatology. 2011;54:452–62. doi: 10.1002/hep.24349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Papeleu P, Vanhaecke T, Henkens T, Elaut G, Vinken M, Snykers S, et al. Isolation of rat hepatocytes. Methods Mol Biol. 2006;320:229–37. doi: 10.1385/1-59259-998-2:229. [DOI] [PubMed] [Google Scholar]
- [207].Tang Y, Bian Z, Zhao L, Liu Y, Liang S, Wang Q, et al. Interleukin-17 exacerbates hepatic steatosis and inflammation in non-alcoholic fatty liver disease. Clin Exp Immunol. 2011;166:281–90. doi: 10.1111/j.1365-2249.2011.04471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].De Gottardi A, Spahr L, Ravier-Dall’Antonia F, Hadengue A. Cannabinoid receptor 1 and 2 agonists increase lipid accumulation in hepatocytes. Liver Int. 2010;30:1482–9. doi: 10.1111/j.1478-3231.2010.02298.x. [DOI] [PubMed] [Google Scholar]
- [209].Gómez-Lechón MJ, Donato MT, Martínez-Romero A, Jiménez N, Castell JV, O’Connor JE. A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact. 2007;165:106–16. doi: 10.1016/j.cbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- [210].Fujimura H, Murakami N, Kurabe M, Toriumi W. In vitro assay for drug-induced hepatosteatosis using rat primary hepatocytes, a fluorescent lipid analog and gene expression analysis. J Appl Toxicol. 2009;29:356–63. doi: 10.1002/jat.1420. [DOI] [PubMed] [Google Scholar]
- [211].Guguen-Guillouzo C, Guillouzo A. General review on in vitro hepatocyte models and their applications. Methods Mol Biol. 2010;640:1–40. doi: 10.1007/978-1-60761-688-7_1. [DOI] [PubMed] [Google Scholar]
- [212].Donato MT, Jiménez N, Serralta A, Mir J, Castell JV, Gómez-Lechón MJ. Effects of steatosis on drug-metabolizing capability of primary human hepatocytes. Toxicol In Vitro. 2007;21:271–6. doi: 10.1016/j.tiv.2006.07.008. [DOI] [PubMed] [Google Scholar]
- [213].Lin Y, Smit MJ, Havinga R, Verkade HJ, Vonk RJ, Kuipers F. Differential effects of eicosapentaenoic acid on glycerolipid and apolipoprotein B metabolism in primary human hepatocytes compared to HepG2 cells and primary rat hepatocytes. Biochim Biophys Acta. 1995;1256:88–96. doi: 10.1016/0005-2760(95)00006-x. [DOI] [PubMed] [Google Scholar]
- [214].Donato MT, Jover R, Gómez-Lechón MJ. Hepatic cell lines for drug hepatotoxicity testing: limitations and strategies to upgrade their metabolic competence by gene engineering. Curr Drug Metab. 2013;14:946–68. doi: 10.2174/1389200211314090002. [DOI] [PubMed] [Google Scholar]
- [215].Knowles BB, Howe CC, Aden DP. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–9. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- [216].Dashti N, Wolfbauer G. Secretion of lipids, apolipoproteins, and lipoproteins by human hepatoma cell line, HepG2: effects of oleic acid and insulin. J Lipid Res. 1987;28:423–36. [PubMed] [Google Scholar]
- [217].Shan X, Miao Y, Fan R, Song C, Wu G, Wan Z, et al. Suppression of Grb2 expression improved hepatic steatosis, oxidative stress, and apoptosis induced by palmitic acid in vitro partly through insulin signaling alteration. In Vitro Cell Dev Biol Anim. 2013;49:576–82. doi: 10.1007/s11626-013-9646-9. [DOI] [PubMed] [Google Scholar]
- [218].Zhao F, Xie P, Jiang J, Zhang L, An W, Zhan Y. The effect and mechanism of tamoxifen-induced hepatocyte steatosis in vitro. Int J Mol Sci. 2014;15:4019–30. doi: 10.3390/ijms15034019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Garcia MC, Amankwa-Sakyi M, Flynn TJ. Cellular glutathione in fatty liver in vitro models. Toxicol In Vitro. 2011;25:1501–16. doi: 10.1016/j.tiv.2011.05.011. [DOI] [PubMed] [Google Scholar]
- [220].Anthérieu S, Rogue A, Fromenty B, Guillouzo A, Robin MA. Induction of vesicular steatosis by amiodarone and tetracycline is associated with up-regulation of lipogenic genes in HepaRG cells. Hepatology. 2011;53:1895–905. doi: 10.1002/hep.24290. [DOI] [PubMed] [Google Scholar]
- [221].Madec S, Cerec V, Plée-Gautier E, Antoun J, Glaise D, Salaun JP, et al. CYP4F3B expression is associated with differentiation of HepaRG human hepatocytes and unaffected by fatty acid overload. Drug Metab Dispos. 2011;39:1987–96. doi: 10.1124/dmd.110.036848. [DOI] [PubMed] [Google Scholar]
- [222].Chavez-Tapia NC, Rosso N, Tiribelli C. Effect of intracellular lipid accumulation in a new model of non-alcoholic fatty liver disease. BMC Gastroenterol. 2012;12:20. doi: 10.1186/1471-230X-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Malhi H, Barreyro FJ, Isomoto H, Bronk SF, Gores GJ. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–31. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Bhatia SN, Balis UJ, Yarmush ML, Toner M. Effect of cell-cell interactions in preservation of cellular phenotype: cocultivation of hepatocytes and nonparenchymal cells. FASEB J. 1999;13:1883–900. doi: 10.1096/fasebj.13.14.1883. [DOI] [PubMed] [Google Scholar]
- [225].Krause P, Saghatolislam F, Koenig S, Unthan-Fechner K, Probst I. Maintaining hepatocyte differentiation in vitro through co-culture with hepatic stellate cells. In Vitro Cell Dev Biol Anim. 2009;45:205–12. doi: 10.1007/s11626-008-9166-1. [DOI] [PubMed] [Google Scholar]
- [226].Khetani SR, Bhatia SN. Microscale culture of human liver cells for drug development. Nat Biotechnol. 2008;26:120–6. doi: 10.1038/nbt1361. [DOI] [PubMed] [Google Scholar]
- [227].Giraudi PJ, Barbero Becerra VJ, Marin V, Chavez-Tapia NC, Tiribelli C, Rosso N. The importance of the interaction between hepatocyte and hepatic stellate cells in fibrogenesis induced by fatty accumulation. Exp Mol Pathol. 2014;98:85–92. doi: 10.1016/j.yexmp.2014.12.006. [DOI] [PubMed] [Google Scholar]
- [228].Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Büttner R, et al. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19:996–1005. doi: 10.1038/cr.2009.73. [DOI] [PubMed] [Google Scholar]
- [229].Bhatia SN, Yarmush ML, Toner M. Controlling cell interactions by micropatterning in co-cultures: hepatocytes and 3T3 fibroblasts. J Biomed Mater Res. 1997;34:189–99. doi: 10.1002/(sici)1097-4636(199702)34:2<189::aid-jbm8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- [230].Ukairo O, Kanchagar C, Moore A, Shi J, Gaffney J, Aoyama S, et al. Long-term stability of primary rat hepatocytes in micropatterned cocultures. J Biochem Mol Toxicol. 2013;27:204–12. doi: 10.1002/jbt.21469. [DOI] [PubMed] [Google Scholar]
- [231].Khetani SR, Kanchagar C, Ukairo O, Krzyzewski S, Moore A, Shi J, et al. Use of micropatterned cocultures to detect compounds that cause drug-induced liver injury in humans. Toxicol Sci. 2013;132:107–17. doi: 10.1093/toxsci/kfs326. [DOI] [PubMed] [Google Scholar]
- [232].Davidson MD, Lehrer M, Khetani SR. Hormone and Drug-Mediated Modulation of Glucose Metabolism in a Microscale Model of the Human Liver. Tissue Eng Part C Methods. 2014 doi: 10.1089/ten.tec.2014.0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Lin C, Ballinger KR, Khetani SR. The application of engineered liver tissues for novel drug discovery. Expert Opin Drug Discov. 2015;10:519–40. doi: 10.1517/17460441.2015.1032241. [DOI] [PubMed] [Google Scholar]
- [234].Godoy P, Hewitt NJ, Albrecht U, Andersen ME, Ansari N, Bhattacharya S, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87:1315–530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Nativ NI, Yarmush G, Chen A, Dong D, Henry SD, Guarrera JV, et al. Rat hepatocyte culture model of macrosteatosis: effect of macrosteatosis induction and reversal on viability and liver-specific function. J Hepatol. 2013;59:1307–14. doi: 10.1016/j.jhep.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Parmentier C, Truisi GL, Moenks K, Stanzel S, Lukas A, Kopp-Schneider A, et al. Transcriptomic hepatotoxicity signature of chlorpromazine after short- and long-term exposure in primary human sandwich cultures. Drug Metab Dispos. 2013;41:1835–42. doi: 10.1124/dmd.113.052415. [DOI] [PubMed] [Google Scholar]
- [237].Surendradoss J, Chang TK, Abbott FS. Evaluation of In Situ Generated Valproyl 1-O-β-Acyl Glucuronide in Valproic Acid Toxicity in Sandwich-Cultured Rat Hepatocytes. Drug Metab Dispos. 2014 doi: 10.1124/dmd.114.059352. [DOI] [PubMed] [Google Scholar]
- [238].Janorkar AV, Harris LM, Murphey BS, Sowell BL. Use of three-dimensional spheroids of hepatocyte-derived reporter cells to study the effects of intracellular fat accumulation and subsequent cytokine exposure. Biotechnol Bioeng. 2011;108:1171–80. doi: 10.1002/bit.23025. [DOI] [PubMed] [Google Scholar]
- [239].Damelin LH, Coward S, Kirwan M, Collins P, Selden C, Hodgson HJ. Fat-loaded HepG2 spheroids exhibit enhanced protection from Pro-oxidant and cytokine induced damage. J Cell Biochem. 2007;101:723–34. doi: 10.1002/jcb.21229. [DOI] [PubMed] [Google Scholar]
- [240].Lu Y, Zhang G, Shen C, Uygun K, Yarmush ML, Meng Q. A novel 3D liver organoid system for elucidation of hepatic glucose metabolism. Biotechnol Bioeng. 2012;109:595–604. doi: 10.1002/bit.23349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Graaf IA, Groothuis GM, Olinga P. Precision-cut tissue slices as a tool to predict metabolism of novel drugs. Expert Opin Drug Metab Toxicol. 2007;3:879–98. doi: 10.1517/17425255.3.6.879. [DOI] [PubMed] [Google Scholar]
- [242].Green CJ, Pramfalk C, Morten KJ, Hodson L. From whole body to cellular models of hepatic triglyceride metabolism: man has got to know his limitations. Am J Physiol Endocrinol Metab. 2015;308:E1–20. doi: 10.1152/ajpendo.00192.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Szalowska E, van der Burg B, Man HY, Hendriksen PJ, Peijnenburg AA. Model steatogenic compounds (amiodarone, valproic acid, and tetracycline) alter lipid metabolism by different mechanisms in mouse liver slices. PLoS One. 2014;9:e86795. doi: 10.1371/journal.pone.0086795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Nassir F, Adewole OL, Brunt EM, Abumrad NA. CD36 deletion reduces VLDL secretion, modulates liver prostaglandins, and exacerbates hepatic steatosis in ob/ob mice. J Lipid Res. 2013;54:2988–97. doi: 10.1194/jlr.M037812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Tryndyak V, Kovalchuk O, Pogribny IP. Identification of differentially methylated sites within unmethylated DNA domains in normal and cancer cells. Anal Biochem. 2006;356:202–7. doi: 10.1016/j.ab.2006.05.019. [DOI] [PubMed] [Google Scholar]
- [246].Jun HJ, Kim J, Hoang MH, Lee SJ. Hepatic lipid accumulation alters global histone h3 lysine 9 and 4 trimethylation in the peroxisome proliferator-activated receptor alpha network. PLoS One. 2012;7:e44345. doi: 10.1371/journal.pone.0044345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Pirola CJ, Gianotti TF, Burgueño AL, Rey-Funes M, Loidl CF, Mallardi P, et al. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut. 2013;62:1356–63. doi: 10.1136/gutjnl-2012-302962. [DOI] [PubMed] [Google Scholar]
- [248].Sookoian S, Rosselli MS, Gemma C, Burgueño AL, Fernández Gianotti T, Castaño GO, et al. Epigenetic regulation of insulin resistance in nonalcoholic fatty liver disease: impact of liver methylation of the peroxisome proliferator-activated receptor γ coactivator 1α promoter. Hepatology. 2010;52:1992–2000. doi: 10.1002/hep.23927. [DOI] [PubMed] [Google Scholar]
- [249].Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- [250].Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–36. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–81. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- [252].Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, et al. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–20. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Pirola CJ, Fernández Gianotti T, Castaño GO, Mallardi P, San Martino J, Mora Gonzalez Lopez Ledesma M, et al. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2014 doi: 10.1136/gutjnl-2014-306996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [254].Yamada H, Suzuki K, Ichino N, Ando Y, Sawada A, Osakabe K, et al. Associations between circulating microRNAs (miR-21, miR-34a, miR-122 and miR-451) and non-alcoholic fatty liver. Clin Chim Acta. 2013;424:99–103. doi: 10.1016/j.cca.2013.05.021. [DOI] [PubMed] [Google Scholar]
- [255].Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;6:e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871–83. doi: 10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Mattis AN, Song G, Hitchner K, Kim RY, Lee AY, Sharma AD, et al. A screen in mice uncovers repression of lipoprotein lipase by microRNA-29a as a mechanism for lipid distribution away from the liver. Hepatology. 2014 doi: 10.1002/hep.27379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Pogribny IP, Starlard-Davenport A, Tryndyak VP, Han T, Ross SA, Rusyn I, et al. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab Invest. 2010;90:1437–46. doi: 10.1038/labinvest.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Alisi A, Da Sacco L, Bruscalupi G, Piemonte F, Panera N, De Vito R, et al. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab Invest. 2011;91:283–93. doi: 10.1038/labinvest.2010.166. [DOI] [PubMed] [Google Scholar]
- [260].Vella S, Gnani D, Crudele A, Ceccarelli S, De Stefanis C, Gaspari S, et al. EZH2 down-regulation exacerbates lipid accumulation and inflammation in in vitro and in vivo NAFLD. Int J Mol Sci. 2013;14:24154–68. doi: 10.3390/ijms141224154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Lee J, Kemper JK. Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging (Albany NY) 2010;2:527–34. doi: 10.18632/aging.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, et al. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474:649–53. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- [263].Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, et al. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;12:1062–72. doi: 10.1111/acel.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ, CRN N. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Liu YL, Patman GL, Leathart JB, Piguet AC, Burt AD, Dufour JF, et al. Carriage of the PNPLA3 rs738409 C >G polymorphism confers an increased risk of non-alcoholic fatty liver disease associated hepatocellular carcinoma. J Hepatol. 2014;61:75–81. doi: 10.1016/j.jhep.2014.02.030. [DOI] [PubMed] [Google Scholar]
- [267].Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JB, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Kiziltas S, Ata P, Colak Y, Mesçi B, Senates E, Enc F, et al. TLR4 gene polymorphism in patients with nonalcoholic fatty liver disease in comparison to healthy controls. Metab Syndr Relat Disord. 2014;12:165–70. doi: 10.1089/met.2013.0120. [DOI] [PubMed] [Google Scholar]
- [269].Musso G, Bo S, Cassader M, De Michieli F, Gambino R. Impact of sterol regulatory element-binding factor-1c polymorphism on incidence of nonalcoholic fatty liver disease and on the severity of liver disease and of glucose and lipid dysmetabolism. Am J Clin Nutr. 2013;98:895–906. doi: 10.3945/ajcn.113.063792. [DOI] [PubMed] [Google Scholar]
- [270].Zain SM, Mohamed Z, Mohamed R. A common variant in the glucokinase regulatory gene rs780094 and risk of nonalcoholic fatty liver disease: A meta-analysis. J Gastroenterol Hepatol. 2015;30:21–7. doi: 10.1111/jgh.12714. [DOI] [PubMed] [Google Scholar]
- [271].Zheng W, Wang L, Su X, Hu XF. MTP -493G>T polymorphism and susceptibility to nonalcoholic fatty liver disease: a meta-analysis. DNA Cell Biol. 2014;33:361–9. doi: 10.1089/dna.2013.2238. [DOI] [PubMed] [Google Scholar]
- [272].Petta S, Miele L, Bugianesi E, Cammà C, Rosso C, Boccia S, et al. Glucokinase regulatory protein gene polymorphism affects liver fibrosis in non-alcoholic fatty liver disease. PLoS One. 2014;9:e87523. doi: 10.1371/journal.pone.0087523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Li MR, Zhang SH, Chao K, Liao XH, Yao JY, Chen MH, et al. Apolipoprotein C3 (-455T>C) polymorphism confers susceptibility to nonalcoholic fatty liver disease in the Southern Han Chinese population. World J Gastroenterol. 2014;20:14010–7. doi: 10.3748/wjg.v20.i38.14010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [274].Wood SL, Westbrook JA, Brown JE. Omic-profiling in breast cancer metastasis to bone: implications for mechanisms, biomarkers and treatment. Cancer Treat Rev. 2014;40:139–52. doi: 10.1016/j.ctrv.2013.07.006. [DOI] [PubMed] [Google Scholar]
- [275].Guillén N, Navarro MA, Arnal C, Noone E, Arbonés-Mainar JM, Acín S, et al. Microarray analysis of hepatic gene expression identifies new genes involved in steatotic liver. Physiol Genomics. 2009;37:187–98. doi: 10.1152/physiolgenomics.90339.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [276].Hennig EE, Mikula M, Goryca K, Paziewska A, Ledwon J, Nesteruk M, et al. Extracellular matrix and cytochrome P450 gene expression can distinguish steatohepatitis from steatosis in mice. J Cell Mol Med. 2014;18:1762–72. doi: 10.1111/jcmm.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Xu E, Forest MP, Schwab M, Avramoglu RK, St-Amand E, Caron AZ, et al. Hepatocyte-specific Ptpn6 deletion promotes hepatic lipid accretion, but reduces NAFLD in diet-induced obesity: potential role of PPARγ. Hepatology. 2014;59:1803–15. doi: 10.1002/hep.26957. [DOI] [PubMed] [Google Scholar]
- [278].Chiappini F, Barrier A, Saffroy R, Domart MC, Dagues N, Azoulay D, et al. Exploration of global gene expression in human liver steatosis by high-density oligonucleotide microarray. Lab Invest. 2006;86:154–65. doi: 10.1038/labinvest.3700374. [DOI] [PubMed] [Google Scholar]
- [279].Starmann J, Fälth M, Spindelböck W, Lanz KL, Lackner C, Zatloukal K, et al. Gene expression profiling unravels cancer-related hepatic molecular signatures in steatohepatitis but not in steatosis. PLoS One. 2012;7:e46584. doi: 10.1371/journal.pone.0046584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Luo L, Dong LY, Yan QG, Cao SJ, Wen XT, Huang Y, et al. Research progress in applying proteomics technology to explore early diagnosis biomarkers of breast cancer, lung cancer and ovarian cancer. Asian Pac J Cancer Prev. 2014;15:8529–38. doi: 10.7314/apjcp.2014.15.20.8529. [DOI] [PubMed] [Google Scholar]
- [281].Zhang L, Perdomo G, Kim DH, Qu S, Ringquist S, Trucco M, et al. Proteomic analysis of fructose-induced fatty liver in hamsters. Metabolism. 2008;57:1115–24. doi: 10.1016/j.metabol.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Park JE, Kim HT, Lee S, Lee YS, Choi UK, Kang JH, et al. Differential expression of intermediate filaments in the process of developing hepatic steatosis. Proteomics. 2011;11:2777–89. doi: 10.1002/pmic.201000544. [DOI] [PubMed] [Google Scholar]
- [283].Hui X, Zhu W, Wang Y, Lam KS, Zhang J, Wu D, et al. Major urinary protein-1 increases energy expenditure and improves glucose intolerance through enhancing mitochondrial function in skeletal muscle of diabetic mice. J Biol Chem. 2009;284:14050–7. doi: 10.1074/jbc.M109.001107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Suski M, Olszanecki R, Madej J, Totoń-Żurańska J, Niepsuj A, Jawień J, et al. Proteomic analysis of changes in protein expression in liver mitochondria in apoE knockout mice. J Proteomics. 2011;74:887–93. doi: 10.1016/j.jprot.2011.03.003. [DOI] [PubMed] [Google Scholar]
- [285].Thomas A, Stevens AP, Klein MS, Hellerbrand C, Dettmer K, Gronwald W, et al. Early changes in the liver-soluble proteome from mice fed a nonalcoholic steatohepatitis inducing diet. Proteomics. 2012;12:1437–51. doi: 10.1002/pmic.201100628. [DOI] [PubMed] [Google Scholar]
- [286].Kim GH, Park EC, Yun SH, Hong Y, Lee DG, Shin EY, et al. Proteomic and bioinformatic analysis of membrane proteome in type 2 diabetic mouse liver. Proteomics. 2013;13:1164–79. doi: 10.1002/pmic.201200210. [DOI] [PubMed] [Google Scholar]
- [287].Bondia-Pons I, Boqué N, Paternain L, Santamaría E, Fernández J, Campión J, et al. Liver proteome changes induced by a short-term high-fat sucrose diet in wistar rats. J Nutrigenet Nutrigenomics. 2011;4:344–53. doi: 10.1159/000336075. [DOI] [PubMed] [Google Scholar]
- [288].Charlton M, Viker K, Krishnan A, Sanderson S, Veldt B, Kaalsbeek AJ, et al. Differential expression of lumican and fatty acid binding protein-1: new insights into the histologic spectrum of nonalcoholic fatty liver disease. Hepatology. 2009;49:1375–84. doi: 10.1002/hep.22927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Su W, Wang Y, Jia X, Wu W, Li L, Tian X, et al. Comparative proteomic study reveals 17β-HSD13 as a pathogenic protein in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2014;111:11437–42. doi: 10.1073/pnas.1410741111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [290].Patel S, Ahmed S. Emerging field of metabolomics: Big promise for cancer biomarker identification and drug discovery. J Pharm Biomed Anal. 2014;107C:63–74. doi: 10.1016/j.jpba.2014.12.020. [DOI] [PubMed] [Google Scholar]
- [291].Atherton HJ, Gulston MK, Bailey NJ, Cheng KK, Zhang W, Clarke K, et al. Metabolomics of the interaction between PPAR-alpha and age in the PPAR-alpha-null mouse. Mol Syst Biol. 2009;5:259. doi: 10.1038/msb.2009.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Vinaixa M, Rodríguez MA, Rull A, Beltrán R, Bladé C, Brezmes J, et al. Metabolomic assessment of the effect of dietary cholesterol in the progressive development of fatty liver disease. J Proteome Res. 2010;9:2527–38. doi: 10.1021/pr901203w. [DOI] [PubMed] [Google Scholar]
- [293].Zhang L, Ye Y, An Y, Tian Y, Wang Y, Tang H. Systems responses of rats to aflatoxin B1 exposure revealed with metabonomic changes in multiple biological matrices. J Proteome Res. 2011;10:614–23. doi: 10.1021/pr100792q. [DOI] [PubMed] [Google Scholar]
- [294].Rubio-Aliaga I, Roos B, Sailer M, McLoughlin GA, Boekschoten MV, van Erk M, et al. Alterations in hepatic one-carbon metabolism and related pathways following a high-fat dietary intervention. Physiol Genomics. 2011;43:408–16. doi: 10.1152/physiolgenomics.00179.2010. [DOI] [PubMed] [Google Scholar]
- [295].Macotela Y, Emanuelli B, Bång AM, Espinoza DO, Boucher J, Beebe K, et al. Dietary leucine--an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].García-Heredia A, Kensicki E, Mohney RP, Rull A, Triguero I, Marsillach J, et al. Paraoxonase-1 deficiency is associated with severe liver steatosis in mice fed a high-fat high-cholesterol diet: a metabolomic approach. J Proteome Res. 2013;12:1946–55. doi: 10.1021/pr400050u. [DOI] [PubMed] [Google Scholar]
- [297].Tanaka N, Matsubara T, Krausz KW, Patterson AD, Gonzalez FJ. Disruption of phospholipid and bile acid homeostasis in mice with nonalcoholic steatohepatitis. Hepatology. 2012;56:118–29. doi: 10.1002/hep.25630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [298].Tanaka N, Takahashi S, Fang ZZ, Matsubara T, Krausz KW, Qu A, et al. Role of white adipose lipolysis in the development of NASH induced by methionine- and choline-deficient diet. Biochim Biophys Acta. 2014;1841:1596–607. doi: 10.1016/j.bbalip.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Yamazaki M, Miyake M, Sato H, Masutomi N, Tsutsui N, Adam KP, et al. Perturbation of bile acid homeostasis is an early pathogenesis event of drug induced liver injury in rats. Toxicol Appl Pharmacol. 2013;268:79–89. doi: 10.1016/j.taap.2013.01.018. [DOI] [PubMed] [Google Scholar]
- [300].Li H, Wang L, Yan X, Liu Q, Yu C, Wei H, et al. A proton nuclear magnetic resonance metabonomics approach for biomarker discovery in nonalcoholic fatty liver disease. J Proteome Res. 2011;10:2797–806. doi: 10.1021/pr200047c. [DOI] [PubMed] [Google Scholar]
- [301].Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–13. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Lake AD, Novak P, Shipkova P, Aranibar N, Robertson D, Reily MD, et al. Decreased hepatotoxic bile acid composition and altered synthesis in progressive human nonalcoholic fatty liver disease. Toxicol Appl Pharmacol. 2013;268:132–40. doi: 10.1016/j.taap.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].García-Cañaveras JC, Donato MT, Castell JV, Lahoz A. A comprehensive untargeted metabonomic analysis of human steatotic liver tissue by RP and HILIC chromatography coupled to mass spectrometry reveals important metabolic alterations. J Proteome Res. 2011;10:4825–34. doi: 10.1021/pr200629p. [DOI] [PubMed] [Google Scholar]
- [304].Patel V, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis. 2013;17:533–46. vii. doi: 10.1016/j.cld.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Mayeux R. Biomarkers: potential uses and limitations. NeuroRx. 2004;1:182–8. doi: 10.1602/neurorx.1.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [306].Buettner R, Schölmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity (Silver Spring) 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- [307].Rodrigues RM, De Kock J, Branson S, Vinken M, Meganathan K, Chaudhari U, et al. Human skin-derived stem cells as a novel cell source for in vitro hepatotoxicity screening of pharmaceuticals. Stem Cells Dev. 2014;23:44–55. doi: 10.1089/scd.2013.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Itoh M, Suganami T, Nakagawa N, Tanaka M, Yamamoto Y, Kamei Y, et al. Melanocortin 4 receptor-deficient mice as a novel mouse model of nonalcoholic steatohepatitis. Am J Pathol. 2011;179:2454–63. doi: 10.1016/j.ajpath.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Reddy JK. Nonalcoholic steatosis and steatohepatitis. III. Peroxisomal beta-oxidation, PPAR alpha, and steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1333–9. doi: 10.1152/ajpgi.2001.281.6.G1333. [DOI] [PubMed] [Google Scholar]
- [310].Nomoto K, Tsuneyama K, Abdel Aziz HO, Takahashi H, Murai Y, Cui ZG, et al. Disrupted galectin-3 causes non-alcoholic fatty liver disease in male mice. J Pathol. 2006;210:469–77. doi: 10.1002/path.2065. [DOI] [PubMed] [Google Scholar]
- [311].Ibdah JA, Paul H, Zhao Y, Binford S, Salleng K, Cline M, et al. Lack of mitochondrial trifunctional protein in mice causes neonatal hypoglycemia and sudden death. J Clin Invest. 2001;107:1403–9. doi: 10.1172/JCI12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Ibdah JA, Perlegas P, Zhao Y, Angdisen J, Borgerink H, Shadoan MK, et al. Mice heterozygous for a defect in mitochondrial trifunctional protein develop hepatic steatosis and insulin resistance. Gastroenterology. 2005;128:1381–90. doi: 10.1053/j.gastro.2005.02.001. [DOI] [PubMed] [Google Scholar]
- [313].Fan CY, Pan J, Chu R, Lee D, Kluckman KD, Usuda N, et al. Hepatocellular and hepatic peroxisomal alterations in mice with a disrupted peroxisomal fatty acyl-coenzyme A oxidase gene. J Biol Chem. 1996;271:24698–710. doi: 10.1074/jbc.271.40.24698. [DOI] [PubMed] [Google Scholar]
- [314].Horie Y, Suzuki A, Kataoka E, Sasaki T, Hamada K, Sasaki J, et al. Hepatocyte-specific Pten deficiency results in steatohepatitis and hepatocellular carcinomas. J Clin Invest. 2004;113:1774–83. doi: 10.1172/JCI20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Martínez-Chantar ML, Corrales FJ, Martínez-Cruz LA, García-Trevijano ER, Huang ZZ, Chen L, et al. Spontaneous oxidative stress and liver tumors in mice lacking methionine adenosyltransferase 1A. FASEB J. 2002;16:1292–4. doi: 10.1096/fj.02-0078fje. [DOI] [PubMed] [Google Scholar]
- [316].Liu Y, Michael MD, Kash S, Bensch WR, Monia BP, Murray SF, et al. Deficiency of adiponectin receptor 2 reduces diet-induced insulin resistance but promotes type 2 diabetes. Endocrinology. 2007;148:683–92. doi: 10.1210/en.2006-0708. [DOI] [PubMed] [Google Scholar]
- [317].Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–9. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- [318].Matthews VB, Allen TL, Risis S, Chan MH, Henstridge DC, Watson N, et al. Interleukin-6-deficient mice develop hepatic inflammation and systemic insulin resistance. Diabetologia. 2010;53:2431–41. doi: 10.1007/s00125-010-1865-y. [DOI] [PubMed] [Google Scholar]
- [319].Kong B, Luyendyk JP, Tawfik O, Guo GL. Farnesoid X receptor deficiency induces nonalcoholic steatohepatitis in low-density lipoprotein receptor-knockout mice fed a high-fat diet. J Pharmacol Exp Ther. 2009;328:116–22. doi: 10.1124/jpet.108.144600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [320].Goel R, Boylan B, Gruman L, Newman PJ, North PE, Newman DK. The proinflammatory phenotype of PECAM-1-deficient mice results in atherogenic diet-induced steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1205–14. doi: 10.1152/ajpgi.00157.2007. [DOI] [PubMed] [Google Scholar]
- [321].Robert K, Nehmé J, Bourdon E, Pivert G, Friguet B, Delcayre C, et al. Cystathionine beta synthase deficiency promotes oxidative stress, fibrosis, and steatosis in mice liver. Gastroenterology. 2005;128:1405–15. doi: 10.1053/j.gastro.2005.02.034. [DOI] [PubMed] [Google Scholar]
- [322].Gary-Bobo M, Elachouri G, Gallas JF, Janiak P, Marini P, Ravinet-Trillou C, et al. Rimonabant reduces obesity-associated hepatic steatosis and features of metabolic syndrome in obese Zucker fa/fa rats. Hepatology. 2007;46:122–9. doi: 10.1002/hep.21641. [DOI] [PubMed] [Google Scholar]
- [323].Watkins SM, Reifsnyder PR, Pan HJ, German JB, Leiter EH. Lipid metabolome-wide effects of the PPARgamma agonist rosiglitazone. J Lipid Res. 2002;43:1809–17. doi: 10.1194/jlr.m200169-jlr200. [DOI] [PubMed] [Google Scholar]
- [324].van Ginneken V, Verhey E, Poelmann R, Ramakers R, van Dijk KW, Ham L, et al. Metabolomics (liver and blood profiling) in a mouse model in response to fasting: a study of hepatic steatosis. Biochim Biophys Acta. 2007;1771:1263–70. doi: 10.1016/j.bbalip.2007.07.007. [DOI] [PubMed] [Google Scholar]