Dynamic Regulation of N-Methyl-d-aspartate (NMDA) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid (AMPA) Receptors by Posttranslational Modifications (original) (raw)
Abstract
Many molecular mechanisms underlie the changes in synaptic glutamate receptor content that are required by neuronal networks to generate cellular correlates of learning and memory. During the last decade, posttranslational modifications have emerged as critical regulators of synaptic transmission and plasticity. Notably, phosphorylation, ubiquitination, and palmitoylation control the stability, trafficking, and synaptic expression of glutamate receptors in the central nervous system. In the current review, we will summarize some of the progress made by the neuroscience community regarding our understanding of phosphorylation, ubiquitination, and palmitoylation of the NMDA and AMPA subtypes of glutamate receptors.
Keywords: alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA receptor, AMPAR); glutamate receptor; post-translational modification (PTM); protein palmitoylation; ubiquitin; N-methyl-D-aspartate receptor (NMDA receptor, NMDAR)
Introduction
The brain functions efficiently due to accurate communication between neurons. At excitatory synapses, the amino acid glutamate is released from synaptic vesicles present in presynaptic terminals; glutamate diffuses into the synaptic cleft and binds to the extracellular region of glutamate receptors (GluRs).2 Glutamate binding to receptors induces structural modification resulting in ion channels opening in the case of ionotropic glutamate receptors (iGluRs) or activation of intracellular signaling cascades upon activation of metabotropic glutamate receptors (mGluRs). Changes in synaptic strength include both potentiation and depression of excitatory neurotransmission, known as long-term potentiation (LTP) and long-term depression (LTD), mechanisms believed to represent cellular correlates of learning and memory (1–3). Over the last three decades, the development of sophisticated biochemical, cellular, and molecular approaches has allowed for in-depth investigation of the detailed mechanisms regulating the content of GluRs at synapses demonstrating that GluRs are dynamic. As shown in Fig. 1, synaptic glutamate receptor localization is regulated by: 1) lateral diffusion to and from synapses; 2) endocytosis and exocytosis at the plasma membrane; and 3) intracellular routing and sorting through endosomal pathways (4–8).
FIGURE 1.
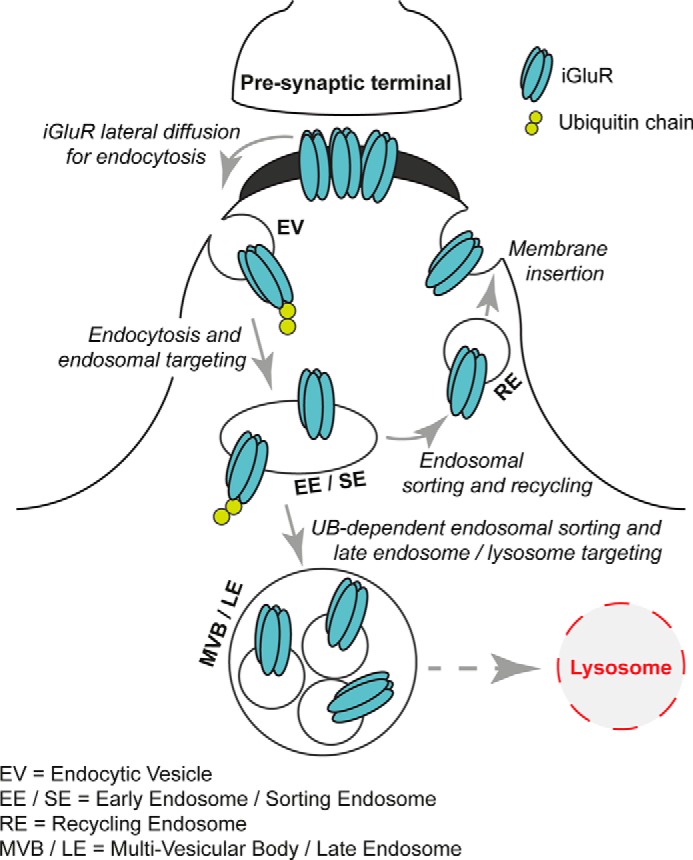
Cellular mechanisms regulating synaptic expression of GluRs. The synaptic molecular content of iGluRs is controlled by multiple cellular events.
It is clear from a multitude of studies that a variety of posttranslational modifications (PTMs) control GluR trafficking and synaptic expression. For example, these modifications play essential roles in influencing protein activity, signaling cascades, protein turnover, synaptic localization, and interactions with intracellular proteins or lipids. These PTMs include glycosylation, phosphorylation, and palmitoylation, which constitute the addition of a functional group to a substrate, and ubiquitination and sumoylation, which involve the covalent conjugation of the protein ubiquitin or the small ubiquitin-like modifier (SUMO) protein to a substrate. Although each of these PTMs can modify GluRs, the current review is specifically focused on the phosphorylation, palmitoylation, and ubiquitination of two subtypes of iGluRs: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) and _N_-methyl-d-aspartate receptors (NMDARs). AMPARs and NMDARs are tetrameric ligand-gated ion channels composed of homologous subunits: AMPARs (GluA1–4) and NMDARs (GluN1; GluN2A-D; GluN3A-B). Each iGluR subunit shares a similar overall topology (Figs. 2 and 3): a long extracellular N-terminal domain (9), a hydrophobic hairpin region forming the pore region that is located between two short intracellular loops (loop1 and loop2), and the first and the second of three membrane-spanning regions. Finally, each subunit has an intracellular C-terminal tail of variable length depending on subtype. The intracellular loops and C-terminal tails have many sites for modifications including palmitoylation, ubiquitination, sumoylation, and phosphorylation, which play critical roles in regulating synaptic expression and intracellular trafficking.
FIGURE 2.
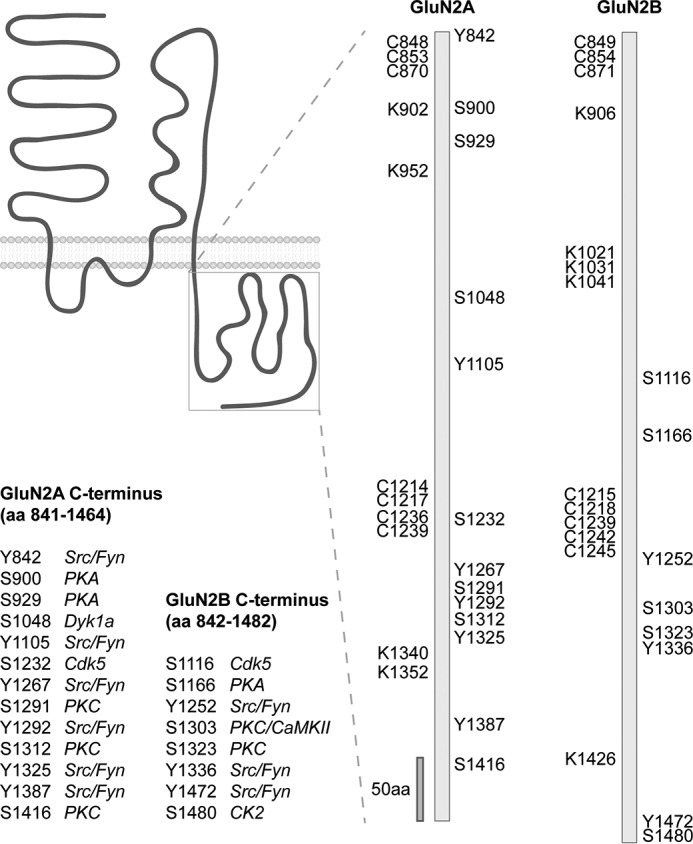
PTMs decorate GluN2A and GluN2B C-terminal tails. The GluN2A and GluN2B C termini contain several amino acids (aa) modified by phosphorylation (serine (S) or tyrosine (Y)), ubiquitination (lysine K)), and palmitoylation (cysteine (C)). Kinases targeting a specific residue are illustrated.
FIGURE 3.
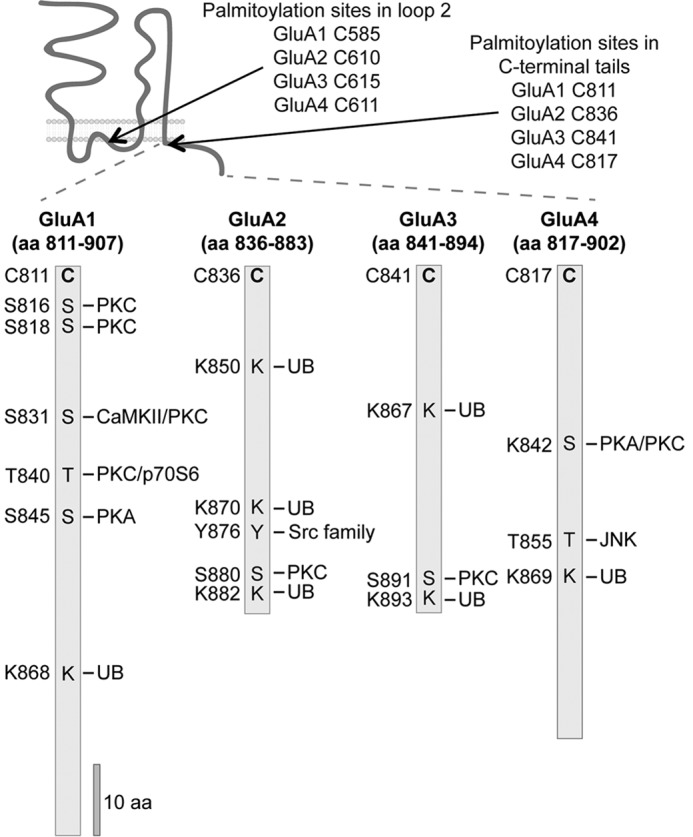
PTMs modify AMPAR intracellular domains. The AMPAR C termini are substrates for several kinases targeting serine (S), threonine (T), or tyrosine (Y). Also, AMPARs are modified by palmitoylation on cysteines (C) and ubiquitination (UB) on lysines (K). The amino acids (aa) targeted by specific PTMs are depicted.
Phosphorylation
Phosphorylation is defined as the reversible addition of a phosphate group (PO43−) to a protein, typically to a Ser, Thr, or Tyr residue, although phosphorylation on His, Arg, or Lys has also been reported (10). The presence of this heavily charged group is important for changing the hydrophobicity and electric charge of a protein region and, therefore, it can result in a change in the protein conformation or interactions with other proteins or cell structures. In the particular case of the GluRs, phosphorylation regulates intracellular trafficking and channel properties. The best-studied example of the latter is for the AMPAR subunit GluA1 in which the conductance and opening probability are modulated by phosphorylation of Ser-831 (PKC/CaMKII) or Ser-845 (PKA), respectively (11, 12). More recently, the phosphorylation on the NMDAR subunit GluN2B by PKA (Ser-1166) was identified as an important factor affecting Ca2+ permeation (13).
One of the most common strategies for studying the effects of phosphorylation is the use of mutants in which the phosphorylation is either blocked or mimicked by replacing the phosphorylated residue with a non-polar amino acid (usually alanine) or a negatively charged one (usually aspartate or glutamate), respectively. This approach has proved powerful and provided valuable information, but it is important to recognize the caveats; it may alter some properties of the protein, masking the effect of the phosphorylation. Therefore, it is not uncommon that both phospho-deficient and phospho-mimetic mutations result in a similar phenotype. For this reason, it is preferable to combine a variety of approaches including biochemical characterization of the mutants and the use of complementary techniques (e.g. pharmacological blockade and/or activation of the kinase). Furthermore, a null phenotype with a phospho-mimetic mutation is not uncommon due to a supposed need for the dynamic on and off of true phosphorylation. One could imagine a protein needing phosphorylation for ER egress, but dephosphorylation for stabilization at the synapse, for example, and a surface expression measure could be confounded.
NMDAR Phosphorylation
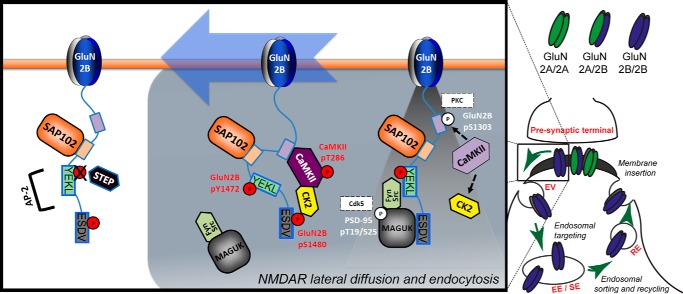
Phosphorylation is a key regulatory mechanism controlling the trafficking of NMDARs (see Fig. 2 for a list of phospho-sites in the GluN2A/2B C termini). Strikingly, phosphorylation regulates the surface and synaptic expression of NMDARs in a subunit-specific manner, providing a highly plastic and precise mechanism to accurately control different subunits in response to stimuli. For example, GluN2B is internalized in response to synaptic activity resulting in reduced surface expression (Fig. 4). Internalization from the plasma membrane is mediated by clathrin and tightly controlled by the phosphorylation of GluN2B on Tyr-1472 by Fyn/Src kinases. This residue is part of the YEKL endocytic motif that is recognized by the clathrin adaptor AP-2 as a required step to induce GluN2B internalization. GluN2B Tyr-1472 phosphorylation blocks AP-2 binding, thus preventing the endocytosis of the receptor and, therefore, increasing its surface expression (14–16). Fyn/Src can directly bind to the family of membrane-associated guanylate kinase (MAGUK) proteins, including PSD-95 and SAP102. Therefore, GluN2B phosphorylation on Tyr-1472 is promoted by the interaction of the receptor with these scaffolding proteins and, consistently, there is elevated phosphorylation of GluN2B on Tyr-1472 associated with synaptic GluN2B. The phosphorylation of GluN2B Ser-1480 by casein kinase 2 (CK2) inversely controls the phosphorylation of GluN2B Tyr-1472. GluN2B Ser-1480 phosphorylation occurs within the PDZ ligand and disrupts binding of the receptor with MAGUK proteins (17). Therefore, phosphorylation of GluN2B on Ser-1480 disrupts anchoring with the postsynaptic density and allows NMDARs to diffuse laterally to extrasynaptic sites corresponding to dephosphorylation of Tyr-1472 by the action of the phosphatase STEP (18). In addition, the disruption of the PDZ ligand “uncouples” the receptor and Fyn/Src kinases, decreasing phosphorylation of Tyr-1472. Therefore, phosphorylation of GluN2B on Ser-1480 results in a decrease in Tyr-1472 phosphorylation, thus promoting internalization (16). A third phosphorylation site on GluN2B is involved in regulating synaptic expression of NMDARs: Ser-1303 by CaMKII/PKC. It is known that synaptic activity enhances the physical interaction of CaMKII with GluN2B (residues 1290–1310) and that phosphorylation of Ser-1303 disrupts CaMKII binding (19). Furthermore, CK2, which phosphorylates the PDZ ligand of GluN2B, can bind to active CaMKII (20). Therefore, CaMKII is able to act as a “scaffolding” protein to couple GluN2B and CK2 in promoting phosphorylation of GluN2B on Ser-1480 (20). Because phosphorylation of Ser-1303 reduces the GluN2B/CaMKII association, it also regulates phosphorylation of Ser-1480 indirectly and ultimately Tyr-1472 phosphorylation. In summary, the phosphorylation of three distinct residues on the cytoplasmic C-tail of GluN2B (Ser-1303, Tyr-1472, and Ser-1480) by four distinct kinases works coordinately to regulate the synaptic expression of GluN2B-containing NMDARs. In addition, another layer of complexity can be added to this mechanism, because the association of MAGUK proteins with Src kinase can be modulated by the Cdk5-mediated phosphorylation of PSD-95 (21). The role of Cdk5 in GluN2B trafficking is more complex; a recent study shows that Cdk5 binds to GluN2B and directly phosphorylates the C terminus on Ser-1116 to decrease receptor surface expression in an activity-dependent manner (22).
FIGURE 4.
NMDAR lateral diffusion and endocytosis. GluN2B/2B receptor removal from synapses is controlled by the coordinated work of several kinases, including CaMKII, CK2, and Fyn/Src. In addition, PKC and Cdk5 may be involved in the process. The synaptic activity-dependent activation of CaMKII promotes phosphorylation on the PDZ ligand of GluN2B by CK2. This phosphorylation disrupts the interaction of the receptor with scaffolding proteins and leads to GluN2B internalization via dephosphorylation of the YEKL endocytic motif. See text for details.
The molecular mechanisms explained above are exclusive for GluN2B-containing NMDARs. GluN2A, the other GluN2 subunit expressed in adult cortex and hippocampus, is subject to differential regulation despite its homology to GluN2B in its C-terminal domain. For example, the PDZ ligand domain of GluN2A is not required for maintaining GluN2A synaptic localization and GluN2A does not interact with CaMKII. Similarly, GluN2A is mainly sorted to degradation after internalization, whereas GluN2B is recycled to the plasma membrane (8). Therefore, from a functional perspective, it is not surprising that GluN2A is not phosphorylated in its PDZ ligand or affected by CaMKII phosphorylation. However, based on the high degree of sequence identity and the close proximity of any kinases to both GluN2B and GluN2A, the lack of phosphorylation is striking. It certainly serves as a cautionary example as to the pitfalls of relying on sequence motifs to predict regulation by phosphorylation.
There are examples of GluN2A being regulated by phosphorylation, although upstream of the extreme C terminus, which proves to be so critical for GluN2B regulation. For example, the kinase Dyrk1a phosphorylates GluN2A in its C-terminal domain, specifically on Ser-1048, and increases GluN2A surface expression by impairing internalization (23). Unfortunately, the molecular mechanisms regulating this process and the physiological significance of this phosphorylation remain unexplored. However, the fact that significant overexpression of Dyrk1a has been observed in a Down syndrome patient raises the possibility of a role for phosphorylation of GluN2A on Ser-1048 in that disease (23). Similarly, the internalization of GluN3A is also controlled by phosphorylation because a recent study identified the phosphorylation of Tyr-971 on GluN3A, within a novel endocytic domain (Tyr-971,Trp-972, and Leu-973). Strikingly, Tyr-971 phosphorylation promotes the interaction with AP-2 and induces GluN3A internalization, just the opposite effect observed for Tyr-1472 on GluN2B (24).
Phosphorylation can modulate receptor surface expression by regulating endocytosis as described above, but also by affecting protein export from the ER to the plasma membrane. For example, phosphorylation on the obligatory NMDAR subunit GluN1 controls the export of newly assembled receptors to the plasma membrane. Specifically, the PKC- and PKA-dependent phosphorylation of Ser-896 and Ser-897, respectively, on GluN1 promotes the release of the receptor from ER to plasma membrane, most probably by masking the adjacent ER retention motif R_X_R (893–895) (25). Similarly, the surface expression of GluN2C in cerebellar granular cells is elevated by PKB/Akt-mediated phosphorylation on Ser-1096. This phosphorylation is activity- and growth factor stimulation-dependent and promotes the association of GluN2C with the adapter protein 14-3-3ϵ. Because 14-3-3ϵ mediates protein export from the ER, phosphorylation of GluN2C on Ser-1096 promotes GluN2C surface expression (26). Interestingly, Ser-1096 on GluN2C is analogous to the CaMKII site, Ser-1303, on GluN2B. Although these two analogous residues on different GluN2 subunits are phosphorylated and functionally important, they have divergent consensus sequences resulting in differing kinase specificities.
AMPAR Phosphorylation
Since the mid-1990s, the cytosolic C-tails of AMPAR subunits have been shown to be targets of a variety of kinases (12, 27–31), which regulate AMPARs in many important ways including endocytosis, intracellular trafficking, channel conductance, and synaptic plasticity (11, 12, 31–34). As shown in Fig. 3, all four AMPAR subunits (GluA1–4) are demonstrated substrates of least one of the following kinases: CaMKII, Fyn, JNK, PKA, PKC, and PKG (5, 35). However, most of our knowledge regarding AMPAR phosphorylation is limited to GluA1 and GluA2, which are widely distributed in the brain. Indeed, GluA1/2 heteromers constitute the majority of AMPARs in the hippocampus (36, 37).
GluA1 was the first AMPAR subunit for which the phosphorylation of the C-terminal tail (Fig. 3) was identified at Ser-831 and Ser-845 (12). Subsequent studies showed that CaMKII specifically phosphorylates Ser-831 (31, 32), which leads to an enhanced single channel conductance (11, 32), whereas the phosphorylation of GluA1 Ser-845 by PKA increases the opening probability of homomeric GluA1 (38). Surprisingly, no interacting partners seem to depend on the phosphorylation state of Ser-845, although Ser-845 phosphorylation regulates recycling (39), whereas Ser-845 dephosphorylation correlates with mechanisms associated with LTD (40, 41). Evidence also suggests that the phosphorylation of GluA2 on Tyr-876 and Ser-880 is essential for receptor endocytosis (42–44). Indeed, GRIP1/2 and PICK1 bind to the extreme GluA2 C-terminal region to the PDZ ligand. Phosphorylation provides elegant specificity of binding as phosphorylation of GluA2 on Tyr-876 and Ser-880 disrupts the binding of GRIP1/2, but is still permissive for PICK1 binding to GluA2 to promote internalization and LTD (43, 45).
It is interesting to note that consensus motifs can be misleading. For example, both PKC and CaMKII recognize very similar sequences (46), but the amino acid sequence surrounding GluA1 Ser-831 does not conform well to the prototypic CaMKII/PKC consensus motif as the residue at position −3 is not basic but hydrophobic (a proline). Thus, it is unclear what other molecular determinants dictate the kinase specificity for Ser-831. In addition to sequence specificity, other factors can modulate receptor phosphorylation such as receptor-binding proteins or other PTMs. For example, SAP97, the only PDZ protein known to bind GluA1, could play a role in regulating PTMs (47). Indeed, a model proposes that SAP97 binds to activated α-CaMKII firmly attached to NMDARs, which provide a solid platform for the synaptic anchoring of newly inserted GluA1-containing AMPARs (48). Thus, SAP97 binding to AMPARs and CaMKII could be a critical mechanism underlying LTP and receptor trafficking (4, 49–55).
Recent studies have revealed the important regulation of GluR trafficking dictated by mechanisms targeting the intracellular loops of GluRs, which include ER retention motifs (56) and residues that are targets for a variety of kinases. Indeed, we found that GluA1 is phosphorylated by CaMKII on Ser-567, a residue in the loop1 region of AMPARs. Surprisingly, phosphorylation on this residue inhibits GluA1-containing AMPAR synaptic insertion under basal condition (57). Instead of promoting AMPAR synaptic expression, the phosphorylation of GluA1 on Ser-567 may represent the first example of an LTD-specific CaMKII substrate that is distinctively different from standard CaMKII substrates such as GluA1 Ser-831 and GluN2B Ser-1303 (58).
It is likely that the AMPAR intracellular loop contains even more regulatory sites, and it seemed unlikely that CaMKII was the only kinase targeting this region. Indeed, in subsequent studies, we found that CK2 phosphorylates the loop1 region of both GluA1 and GluA2 (59). This study shows that blocking phosphorylation of the major CK2 phosphorylation site on GluA1, Ser-579, impairs AMPAR surface and synaptic expression. Interestingly, CK2 can also phosphorylate, at least in vitro, the GluA1 Ser-567 residue previously identified as a CaMKII phosphorylation site (57). Furthermore, casein kinase 1 is another potential kinase that could regulate AMPARs by phosphorylating the loop1 of AMPARs (59). Phosphorylation of this region might not only regulate trafficking, but due to the close proximity to the pore region, could potentially have potent effects on channel properties. Thus, the loop1 of AMPARs may represent an overlooked region with great potential for gaining insight into core mechanisms regulating glutamate receptor function.
Through the years, phosphospecific antibodies, phosphopeptide mapping, mass spectrometry analysis, and genetic approaches generated volumes of data substantiating a critical role for AMPAR phosphorylation in regulating synaptic expression and dynamic AMPAR changes during paradigms of synaptic plasticity. However, a recent study by Hosokawa et al. (60) has attempted to tackle the issue of overall stoichiometry of AMPAR phosphorylation using a different biochemical approach, specifically a Phos-tag SDS-PAGE reagent that resolves molecules by molecular weight, as a reflection of their phosphorylated residues. Thus, the distinction between phosphorylated and not phosphorylated species is possible based on their mobility on SDS-PAGE. Using this technique, 4.3% of all GluA1 found in the hippocampus are phosphorylated at Thr-840, whereas phosphorylated GluA1 at Ser-831 and at Ser-845 represent respectively 0.18% and 0.018% of total GluA1 (60). This estimation is in sharp contrast to other studies that have estimated closer to 15% of surface GluA1 is phosphorylated at Ser-845 under steady-state conditions (61). Furthermore, genetic knock-in approaches have found that GluA1-containing mutations at Ser-831 and Ser-845 display impaired synaptic plasticity (62). Therefore, there are conflicting data, but the study by Hosokawa et al. (60) certainly sheds light on the issue of stoichiometry and how it can be more precisely determined. However, detecting low phosphorylation levels at any given time reflects the transient nature of phosphorylation, and thus studying the stoichiometry of PTMs (i.e. phosphorylation or ubiquitination) on substrates is not necessarily a measure of functional relevance because spatial and temporal resolution is missing.
Ubiquitination
In addition to phosphorylation, other PTMs such as palmitoylation and ubiquitination are gaining attention as well. Indeed, the importance of the ubiquitin (UB) system in regulating virtually all aspects of cell function rivals, and may exceed, the role of protein phosphorylation (63). For example, the UB system preserves cell homeostasis by acting as the primary mechanism of protein quality control, membrane protein trafficking, receptor internalization, and degradation (64, 65).
Ubiquitination is a highly regulated ATP-dependent process that requires the coordinated and sequential action of an E1-activating enzyme, an E2-conjugating enzyme and, finally, an E3 UB ligase. Ultimately, the UB molecule is attached to the substrate via the formation of an isopeptide bond between the C-terminal glycine of UB and an internal lysine within the substrate. The human genome encodes several hundred E3s, and only a few of these have been studied thus far. In mammalian cells, many G protein-coupled receptors and ion channels are ubiquitinated in response to ligand binding (66–74). In addition, the UB-proteasome system influences neuronal activity and glutamatergic neurotransmission. For instance, a study by Ehlers (75) shows that bidirectional homeostatic plasticity triggers activity-dependent ubiquitination and profound modifications of a variety of PSD proteins. This pioneering work, along with studies from other groups, suggests a mechanism for regulating dynamic changes in spine content, morphology, and structure, therefore altering synaptic activity and plasticity (76, 77).
Many studies have identified and characterized the ubiquitination of mammalian iGluRs. For instance, the UB E3 ligases Fbx2 (78) and Mind Bomb-2 (79) ubiquitinate the NMDAR subunits GluN1 and GluN2B in an activity-dependent manner. More recently, GluN2D was shown to be ubiquitinated by Nedd4-1 (80). The ubiquitination of AMPARs, on the other hand, was initially demonstrated in Caenorhabditis elegans (81), and it took another decade before studies demonstrated that mammalian AMPAR subunits were actually ubiquitinated by the UB E3 ligases APCCdh1, Nedd4-1, and RNF167 (68, 69, 72, 73, 82–84). Interestingly, modulation of neuronal activity by repetitive stress induces GluA1 and GluN1 ubiquitination (85). Importantly, two recent proteomic studies performed on rodent brains identified GluN1, GluN2A/2B (Fig. 2), and GluA1–4 (Fig. 3) as being modified by UB (86, 87). Without a doubt, ubiquitination is important for regulating GluRs, but the mechanisms and implications of AMPAR and NMDAR ubiquitination on health and with respect to synaptic dysfunction remain to be investigated in depth.
Palmitoylation
Another common and important PTM that regulates GluR trafficking is palmitoylation. It is defined by the covalent and reversible union of a palmitic acid molecule (saturated 16-carbon lipid) to a cysteine residue in a given protein. The presence of basic and hydrophobic residues surrounding cysteine appears to create a favorable sequence environment for the reaction (88). This likely explains the propensity of palmitoylated cysteines to be identified near the transmembrane-spanning region for membrane proteins (89–92). The addition of the palmitoyl group increases the hydrophobicity of the protein and, therefore, facilitates the interaction with cellular membranes. Palmitoylation can both stabilize proteins in the plasma membrane and control protein shuttling between intracellular compartments (93). Palmitoylation is mediated by a group of enzymes named palmitoyltransferases (PATs), of which there are 23 in humans with each containing an Asp-His-His-Cys (DHHC) Cys-rich domain that confers the molecular signature of PATs (94). Conversely, depalmitoylation is mediated by acyl-protein-thioesterases, of which very few have been identified so far.
The function of many neuronal proteins, including NMDARs and AMPARs, is regulated by palmitoylation. The palmitoylation of GluN2A and GluN2B subunits occurs in “two clusters” (Fig. 2). Cluster I, close to the last transmembrane domain of GluN2A and GluN2B, is associated with an increase in the surface expression of the receptor, whereas palmitoylation of Cluster II, located in the middle of the intracellular C-terminus, plays an opposite role to Cluster I and is associated with receptor accumulation in the Golgi apparatus (95, 96). However, the regulation of NMDARs by palmitoylation is complex and participates in interplay with tyrosine phosphorylation for both GluN2A and GluN2B. In addition, PSD-95 and other synaptic proteins important for controlling NMDARs are also palmitoylated, multiplying the complexity of the regulation of NMDARs by this modification (93). Similarly, all four subunits of AMPARs are palmitoylated at two conserved cysteine residues (Fig. 3): one is located within the C-terminal region, and another is located within the second intracellular loop immediately adjacent to the pore region (89, 97). Although in vitro studies show that AMPAR trafficking and membrane expression are regulated by palmitoylation (95, 97), the study by Van Dolah et al. (98) tackled the function of AMPAR palmitoylation in the brain. In this interesting study, intraperitoneal injection of the psychostimulant cocaine (20 mg/kg) in adult male rats up-regulates the palmitoylation of GluA1 and GluA3 AMPAR subunits in the nucleus accumbens. In fact, cocaine causes the redistribution of AMPARs, increasing the intracellular localization, whereas the surface expression was reduced. Although future studies are required to clarify the function of palmitoylation in AMPAR synaptic plasticity, it is clear that AMPAR palmitoylation can be dynamically controlled by extracellular stimuli in various brain regions (89, 97, 98).
Future Perspectives
Over the last decade, great advances have been made in identifying the specific regulation of AMPARs and NMDARs by PTMs. Several studies demonstrate crosstalk between two or more PTMs to be important mechanisms of synaptic regulation (99). Functionally, crosstalk may occur within the same protein (cis crosstalk) or between PTMs on two different proteins (trans crosstalk). An example of such crosstalk on glutamate receptors is that, after depalmitoylation of GluA1 on Cys-811, the phosphorylation of GluA1 Ser-818 by PKC enhances binding to 4.1N to drive membrane insertion and the expression of LTP (97). As evidenced by this study, future investigations are required for understanding the synergistic/antagonistic effect of PTMs and the directionality of the crosstalk for identifying new mechanisms implicated in spatial and temporal regulation of AMPARs and NMDARs. Interestingly, using mass spectrometry and various enrichment approaches, a recent study suggests the presence of a global crosstalk directionality in which phosphorylation frequently precedes ubiquitination (100). In conclusion, PTMs represent an important set of mechanisms to regulate protein function and cellular signaling, and the importance and complexity of its code remain a major challenge for our complete understanding of brain function.
*
This work was supported by Université du Québec à Montréal Start-up Funds (to M. P. L.) and by National Institutes of Health NIA Grant 1K99AG041225 (to A. S. C.) and the NINDS Intramural Program (to K. W. R.). This is the first article in the Thematic Minireview series “Molecular Mechanisms of Synaptic Plasticity.” The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
2
The abbreviations used are:
GluR
glutamate receptor
iGluR
ionotropic GluR
AMPAR
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
LTP
long-term potentiation
LTD
long-term depression
CaMKII
Ca2+/calmodulin-dependent protein kinase II
CK2
casein kinase 2
NMDAR
NMDA receptor
MAGUK
membrane-associated guanylate kinase
PSD
postsynaptic density
PTM
posttranslational modification
ER
endoplasmic reticulum
UB
ubiquitin.
References
- 1.Bredt D. S., and Nicoll R. A. (2003) AMPA receptor trafficking at excitatory synapses. Neuron 40, 361–379 [DOI] [PubMed] [Google Scholar]
- 2.Malenka R. C., and Bear M. F. (2004) LTP and LTD: an embarrassment of riches. Neuron 44, 5–21 [DOI] [PubMed] [Google Scholar]
- 3.Malinow R., and Malenka R. C. (2002) AMPA receptor trafficking and synaptic plasticity. Annu. Rev. Neurosci. 25, 103–126 [DOI] [PubMed] [Google Scholar]
- 4.Anggono V., and Huganir R. L. (2012) Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 22, 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu W., and Roche K. W. (2012) Posttranslational regulation of AMPA receptor trafficking and function. Curr. Opin. Neurobiol. 22, 470–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Sluijs P., and Hoogenraad C. C. (2011) New insights in endosomal dynamics and AMPA receptor trafficking. Semin. Cell. Dev. Biol. 22, 499–505 [DOI] [PubMed] [Google Scholar]
- 7.Ehlers M. D. (2013) Dendritic trafficking for neuronal growth and plasticity. Biochem. Soc. Trans. 41, 1365–1382 [DOI] [PubMed] [Google Scholar]
- 8.Sanz-Clemente A., Nicoll R. A., and Roche K. W. (2013) Diversity in NMDA receptor composition: many regulators, many consequences. Neuroscientist 19, 62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Standley S., and Baudry M. (2000) The role of glycosylation in ionotropic glutamate receptor ligand binding, function, and trafficking. Cell. Mol. Life Sci. 57, 1508–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cieśla J., Frączyk T., and Rode W. (2011) Phosphorylation of basic amino acid residues in proteins: important but easily missed. Acta Biochim. Pol. 58, 137–148 [PubMed] [Google Scholar]
- 11.Derkach V., Barria A., and Soderling T. R. (1999) Ca2+/calmodulin-kinase II enhances channel conductance of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. U.S.A. 96, 3269–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roche K. W., O'Brien R. J., Mammen A. L., Bernhardt J., and Huganir R. L. (1996) Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron 16, 1179–1188 [DOI] [PubMed] [Google Scholar]
- 13.Murphy J. A., Stein I. S., Lau C. G., Peixoto R. T., Aman T. K., Kaneko N., Aromolaran K., Saulnier J. L., Popescu G. K., Sabatini B. L., Hell J. W., and Zukin R. S. (2014) Phosphorylation of Ser1166 on GluN2B by PKA is critical to synaptic NMDA receptor function and Ca2+ signaling in spines. J. Neurosci. 34, 869–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lavezzari G., McCallum J., Lee R., and Roche K. W. (2003) Differential binding of the AP-2 adaptor complex and PSD-95 to the C-terminus of the NMDA receptor subunit NR2B regulates surface expression. Neuropharmacology 45, 729–737 [DOI] [PubMed] [Google Scholar]
- 15.Prybylowski K., Chang K., Sans N., Kan L., Vicini S., and Wenthold R. J. (2005) The synaptic localization of NR2B-containing NMDA receptors is controlled by interactions with PDZ proteins and AP-2. Neuron 47, 845–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanz-Clemente A., Matta J. A., Isaac J. T., and Roche K. W. (2010) Casein kinase 2 regulates the NR2 subunit composition of synaptic NMDA receptors. Neuron 67, 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung H. J., Huang Y. H., Lau L. F., and Huganir R. L. (2004) Regulation of the NMDA receptor complex and trafficking by activity-dependent phosphorylation of the NR2B subunit PDZ ligand. J. Neurosci. 24, 10248–10259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen B. S., Gray J. A., Sanz-Clemente A., Wei Z., Thomas E. V., Nicoll R. A., and Roche K. W. (2012) SAP102 mediates synaptic clearance of NMDA receptors. Cell Rep. 2, 1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Leary H., Liu W. H., Rorabaugh J. M., Coultrap S. J., and Bayer K. U. (2011) Nucleotides and phosphorylation bi-directionally modulate Ca2+/calmodulin-dependent protein kinase II (CaMKII) binding to the _N_-methyl-d-aspartate (NMDA) receptor subunit GluN2B. J. Biol. Chem. 286, 31272–31281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanz-Clemente A., Gray J. A., Ogilvie K. A., Nicoll R. A., and Roche K. W. (2013) Activated CaMKII couples GluN2B and casein kinase 2 to control synaptic NMDA receptors. Cell Rep. 3, 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morabito M. A., Sheng M., and Tsai L. H. (2004) Cyclin-dependent kinase 5 phosphorylates the N-terminal domain of the postsynaptic density protein PSD-95 in neurons. J. Neurosci. 24, 865–876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plattner F., Hernández A., Kistler T. M., Pozo K., Zhong P., Yuen E. Y., Tan C., Hawasli A. H., Cooke S. F., Nishi A., Guo A., Wiederhold T., Yan Z., and Bibb J. A. (2014) Memory enhancement by targeting Cdk5 regulation of NR2B. Neuron 81, 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grau C., Arató K., Fernández-Fernández J. M., Valderrama A., Sindreu C., Fillat C., Ferrer I., de la Luna S., and Altafaj X. (2014) DYRK1A-mediated phosphorylation of GluN2A at Ser1048 regulates the surface expression and channel activity of GluN1/GluN2A receptors. Front. Cell. Neurosci. 8, 331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowdhury D., Marco S., Brooks I. M., Zandueta A., Rao Y., Haucke V., Wesseling J. F., Tavalin S. J., and Pérez-Otaño I. (2013) Tyrosine phosphorylation regulates the endocytosis and surface expression of GluN3A-containing NMDA receptors. J. Neurosci. 33, 4151–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott D. B., Blanpied T. A., Swanson G. T., Zhang C., and Ehlers M. D. (2001) An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J. Neurosci. 21, 3063–3072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen B. S., and Roche K. W. (2009) Growth factor-dependent trafficking of cerebellar NMDA receptors via protein kinase B/Akt phosphorylation of NR2C. Neuron 62, 471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackstone C., Murphy T. H., Moss S. J., Baraban J. M., and Huganir R. L. (1994) Cyclic AMP and synaptic activity-dependent phosphorylation of AMPA-preferring glutamate receptors. J. Neurosci. 14, 7585–7593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGlade-McCulloh E., Yamamoto H., Tan S. E., Brickey D. A., and Soderling T. R. (1993) Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature 362, 640–642 [DOI] [PubMed] [Google Scholar]
- 29.Moss S. J., Blackstone C. D., and Huganir R. L. (1993) Phosphorylation of recombinant non-NMDA glutamate receptors on serine and tyrosine residues. Neurochem. Res. 18, 105–110 [DOI] [PubMed] [Google Scholar]
- 30.Tan S. E., Wenthold R. J., and Soderling T. R. (1994) Phosphorylation of AMPA-type glutamate receptors by calcium/calmodulin-dependent protein kinase II and protein kinase C in cultured hippocampal neurons. J. Neurosci. 14, 1123–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mammen A. L., Kameyama K., Roche K. W., and Huganir R. L. (1997) Phosphorylation of the α-amino-3-hydroxy-5-methylisoxazole4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem. 272, 32528–32533 [DOI] [PubMed] [Google Scholar]
- 32.Barria A., Muller D., Derkach V., Griffith L. C., and Soderling T. R. (1997) Regulatory phosphorylation of AMPA-type glutamate receptors by CaM-KII during long-term potentiation. Science 276, 2042–2045 [DOI] [PubMed] [Google Scholar]
- 33.Kristensen A. S., Jenkins M. A., Banke T. G., Schousboe A., Makino Y., Johnson R. C., Huganir R., and Traynelis S. F. (2011) Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat. Neurosci. 14, 727–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jenkins M. A., and Traynelis S. F. (2012) PKC phosphorylates GluA1-Ser831 to enhance AMPA receptor conductance. Channels (Austin) 6, 60–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shepherd J. D., and Huganir R. L. (2007) The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 23, 613–643 [DOI] [PubMed] [Google Scholar]
- 36.Lu W., Shi Y., Jackson A. C., Bjorgan K., During M. J., Sprengel R., Seeburg P. H., and Nicoll R. A. (2009) Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron 62, 254–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenthold R. J., Petralia R. S., Blahos J. 2nd, and Niedzielski A. S. (1996) Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 16, 1982–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banke T. G., Bowie D., Lee H., Huganir R. L., Schousboe A., and Traynelis S. F. (2000) Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 20, 89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehlers M. D. (2000) Reinsertion or degradation of AMPA receptors determined by activity-dependent endocytic sorting. Neuron 28, 511–525 [DOI] [PubMed] [Google Scholar]
- 40.Lee H. K., Takamiya K., Han J. S., Man H., Kim C. H., Rumbaugh G., Yu S., Ding L., He C., Petralia R. S., Wenthold R. J., Gallagher M., and Huganir R. L. (2003) Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell 112, 631–643 [DOI] [PubMed] [Google Scholar]
- 41.Brown T. C., Tran I. C., Backos D. S., and Esteban J. A. (2005) NMDA receptor-dependent activation of the small GTPase Rab5 drives the removal of synaptic AMPA receptors during hippocampal LTD. Neuron 45, 81–94 [DOI] [PubMed] [Google Scholar]
- 42.Hayashi T., and Huganir R. L. (2004) Tyrosine phosphorylation and regulation of the AMPA receptor by SRC family tyrosine kinases. J. Neurosci. 24, 6152–6160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chung H. J., Xia J., Scannevin R. H., Zhang X., and Huganir R. L. (2000) Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J. Neurosci. 20, 7258–7267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chung H. J., Steinberg J. P., Huganir R. L., and Linden D. J. (2003) Requirement of AMPA receptor GluR2 phosphorylation for cerebellar long-term depression. Science 300, 1751–1755 [DOI] [PubMed] [Google Scholar]
- 45.Matsuda S., Mikawa S., and Hirai H. (1999) Phosphorylation of serine-880 in GluR2 by protein kinase C prevents its C terminus from binding with glutamate receptor-interacting protein. J. Neurochem. 73, 1765–1768 [DOI] [PubMed] [Google Scholar]
- 46.Rust H. L., and Thompson P. R. (2011) Kinase consensus sequences: a breeding ground for crosstalk. ACS Chem. Biol. 6, 881–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leonard A. S., Davare M. A., Horne M. C., Garner C. C., and Hell J. W. (1998) SAP97 is associated with the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J. Biol. Chem. 273, 19518–19524 [DOI] [PubMed] [Google Scholar]
- 48.Lisman J. E., and Zhabotinsky A. M. (2001) A model of synaptic memory: a CaMKII/PP1 switch that potentiates transmission by organizing an AMPA receptor anchoring assembly. Neuron 31, 191–201 [DOI] [PubMed] [Google Scholar]
- 49.Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., and Waxham M. N. (1989) An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature 340, 554–557 [DOI] [PubMed] [Google Scholar]
- 50.Malinow R., Schulman H., and Tsien R. W. (1989) Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science 245, 862–866 [DOI] [PubMed] [Google Scholar]
- 51.Wyllie D. J., and Nicoll R. A. (1994) A role for protein kinases and phosphatases in the Ca2+-induced enhancement of hippocampal AMPA receptor-mediated synaptic responses. Neuron 13, 635–643 [DOI] [PubMed] [Google Scholar]
- 52.Lisman J., Yasuda R., and Raghavachari S. (2012) Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 13, 169–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kessels H. W., and Malinow R. (2009) Synaptic AMPA receptor plasticity and behavior. Neuron 61, 340–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lüscher C., and Malenka R. C. (2012) NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD). Cold Spring Harb. Perspect. Biol. 4, a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nicoll R. A., and Roche K. W. (2013) Long-term potentiation: peeling the onion. Neuropharmacology 74, 18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nasu-Nishimura Y., Jaffe H., Isaac J. T., and Roche K. W. (2010) Differential regulation of kainate receptor trafficking by phosphorylation of distinct sites on GluR6. J. Biol. Chem. 285, 2847–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu W., Isozaki K., Roche K. W., and Nicoll R. A. (2010) Synaptic targeting of AMPA receptors is regulated by a CaMKII site in the first intracellular loop of GluA1. Proc. Natl. Acad. Sci. U.S.A. 107, 22266–22271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coultrap S. J., Freund R. K., O'Leary H., Sanderson J. L., Roche K. W., Dell'Acqua M. L., and Bayer K. U. (2014) Autonomous CaMKII mediates both LTP and LTD using a mechanism for differential substrate site selection. Cell Rep. 6, 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lussier M. P., Gu X., Lu W., and Roche K. W. (2014) Casein kinase 2 phosphorylates GluA1 and regulates its surface expression. Eur. J. Neurosci. 39, 1148–1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hosokawa T., Mitsushima D., Kaneko R., and Hayashi Y. (2015) Stoichiometry and phosphoisotypes of hippocampal AMPA-type glutamate receptor phosphorylation. Neuron 85, 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oh M. C., Derkach V. A., Guire E. S., and Soderling T. R. (2006) Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J. Biol. Chem. 281, 752–758 [DOI] [PubMed] [Google Scholar]
- 62.Lee H. K., Takamiya K., He K., Song L., and Huganir R. L. (2010) Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J. Neurophysiol. 103, 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Komander D. (2009) The emerging complexity of protein ubiquitination. Biochem. Soc. Trans. 37, 937–953 [DOI] [PubMed] [Google Scholar]
- 64.Ciechanover A., and Iwai K. (2004) The ubiquitin system: from basic mechanisms to the patient bed. IUBMB Life 56, 193–201 [DOI] [PubMed] [Google Scholar]
- 65.Hicke L., and Dunn R. (2003) Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19, 141–172 [DOI] [PubMed] [Google Scholar]
- 66.DiAntonio A., and Hicke L. (2004) Ubiquitin-dependent regulation of the synapse. Annu. Rev. Neurosci. 27, 223–246 [DOI] [PubMed] [Google Scholar]
- 67.Katzmann D. J., Odorizzi G., and Emr S. D. (2002) Receptor downregulation and multivesicular-body sorting. Nat. Rev. Mol. Cell Biol. 3, 893–905 [DOI] [PubMed] [Google Scholar]
- 68.Lussier M. P., Nasu-Nishimura Y., and Roche K. W. (2011) Activity-dependent ubiquitination of the AMPA receptor subunit GluA2. J. Neurosci. 31, 3077–3081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lussier M. P., Herring B. E., Nasu-Nishimura Y., Neutzner A., Karbowski M., Youle R. J., Nicoll R. A., and Roche K. W. (2012) Ubiquitin ligase RNF167 regulates AMPA receptor-mediated synaptic transmission. Proc. Natl. Acad. Sci. U.S.A. 109, 19426–19431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marchese A., and Benovic J. L. (2001) Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J. Biol. Chem. 276, 45509–45512 [DOI] [PubMed] [Google Scholar]
- 71.Rotin D., Staub O., and Haguenauer-Tsapis R. (2000) Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr Biol. 176, 1–17 [DOI] [PubMed] [Google Scholar]
- 72.Scudder S. L., Goo M. S., Cartier A. E., Molteni A., Schwarz L. A., Wright R., and Patrick G. N. (2014) Synaptic strength is bidirectionally controlled by opposing activity-dependent regulation of Nedd4–1 and USP8. J. Neurosci. 34, 16637–16649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwarz L. A., Hall B. J., and Patrick G. N. (2010) Activity-dependent ubiquitination of GluA1 mediates a distinct AMPA receptor endocytosis and sorting pathway. J. Neurosci. 30, 16718–16729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanowitz M., and Von Zastrow M. (2002) Ubiquitination-independent trafficking of G protein-coupled receptors to lysosomes. J. Biol. Chem. 277, 50219–50222 [DOI] [PubMed] [Google Scholar]
- 75.Ehlers M. D. (2003) Ubiquitin and synaptic dysfunction: ataxic mice highlight new common themes in neurological disease. Trends Neurosci. 26, 4–7 [DOI] [PubMed] [Google Scholar]
- 76.DiAntonio A., Haghighi A. P., Portman S. L., Lee J. D., Amaranto A. M., and Goodman C. S. (2001) Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 412, 449–452 [DOI] [PubMed] [Google Scholar]
- 77.Mabb A. M., and Ehlers M. D. (2010) Ubiquitination in postsynaptic function and plasticity. Annu. Rev. Cell Dev. Biol. 26, 179–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kato A. S., Gill M. B., Yu H., Nisenbaum E. S., and Bredt D. S. (2010) TARPs differentially decorate AMPA receptors to specify neuropharmacology. Trends Neurosci. 33, 241–248 [DOI] [PubMed] [Google Scholar]
- 79.Jurd R., Thornton C., Wang J., Luong K., Phamluong K., Kharazia V., Gibb S. L., and Ron D. (2008) Mind bomb-2 is an E3 ligase that ubiquitinates the _N_-methyl-d-aspartate receptor NR2B subunit in a phosphorylation-dependent manner. J. Biol. Chem. 283, 301–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gautam V., Trinidad J. C., Rimerman R. A., Costa B. M., Burlingame A. L., and Monaghan D. T. (2013) Nedd4 is a specific E3 ubiquitin ligase for the NMDA receptor subunit GluN2D. Neuropharmacology 74, 96–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Burbea M., Dreier L., Dittman J. S., Grunwald M. E., and Kaplan J. M. (2002) Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C. elegans. Neuron 35, 107–120 [DOI] [PubMed] [Google Scholar]
- 82.Widagdo J., Chai Y. J., Ridder M. C., Chau Y. Q., Johnson R. C., Sah P., Huganir R. L., and Anggono V. (2015) Activity-dependent ubiquitination of GluA1 and GluA2 regulates AMPA receptor intracellular sorting and degradation. Cell Rep. 10.1016/j.celrep.2015.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin A., Hou Q., Jarzylo L., Amato S., Gilbert J., Shang F., and Man H. Y. (2011) Nedd4-mediated AMPA receptor ubiquitination regulates receptor turnover and trafficking. J. Neurochem. 119, 27–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fu A. K., Hung K. W., Fu W. Y., Shen C., Chen Y., Xia J., Lai K. O., and Ip N. Y. (2011) APCCdh1 mediates EphA4-dependent downregulation of AMPA receptors in homeostatic plasticity. Nat. Neurosci. 14, 181–189 [DOI] [PubMed] [Google Scholar]
- 85.Yuen E. Y., Wei J., Liu W., Zhong P., Li X., and Yan Z. (2012) Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron 73, 962–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wagner S. A., Beli P., Weinert B. T., Schölz C., Kelstrup C. D., Young C., Nielsen M. L., Olsen J. V., Brakebusch C., and Choudhary C. (2012) Proteomic analyses reveal divergent ubiquitylation site patterns in murine tissues. Mol. Cell. Proteomics 11, 1578–1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Na C. H., Jones D. R., Yang Y., Wang X., Xu Y., and Peng J. (2012) Synaptic protein ubiquitination in rat brain revealed by antibody-based ubiquitome analysis. J. Proteome Res. 11, 4722–4732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xue Y., Chen H., Jin C., Sun Z., and Yao X. (2006) NBA-Palm: prediction of palmitoylation site implemented in Naive Bayes algorithm. BMC Bioinformatics 7, 458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hayashi T., Rumbaugh G., and Huganir R. L. (2005) Differential regulation of AMPA receptor subunit trafficking by palmitoylation of two distinct sites. Neuron 47, 709–723 [DOI] [PubMed] [Google Scholar]
- 90.Gauthier-Campbell C., Bredt D. S., Murphy T. H., and El-Husseini A. E. (2004) Regulation of dendritic branching and filopodia formation in hippocampal neurons by specific acylated protein motifs. Mol. Biol. Cell 15, 2205–2217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El-Husseini A. E, and Bredt D. S. (2002) Protein palmitoylation: a regulator of neuronal development and function. Nat. Rev. Neurosci. 3, 791–802 [DOI] [PubMed] [Google Scholar]
- 92.Bijlmakers M. J., and Marsh M. (2003) The on-off story of protein palmitoylation. Trends Cell Biol. 13, 32–42 [DOI] [PubMed] [Google Scholar]
- 93.Fukata Y., and Fukata M. (2010) Protein palmitoylation in neuronal development and synaptic plasticity. Nat. Rev. Neurosci. 11, 161–175 [DOI] [PubMed] [Google Scholar]
- 94.Planey S. L., and Zacharias D. A. (2009) Palmitoyl acyltransferases, their substrates, and novel assays to connect them (Review). Mol. Membr. Biol. 26, 14–31 [DOI] [PubMed] [Google Scholar]
- 95.Hayashi T., Thomas G. M., and Huganir R. L. (2009) Dual palmitoylation of NR2 subunits regulates NMDA receptor trafficking. Neuron 64, 213–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Thomas G. M., and Huganir R. L. (2013) Palmitoylation-dependent regulation of glutamate receptors and their PDZ domain-containing partners. Biochem. Soc. Trans. 41, 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin D. T., Makino Y., Sharma K., Hayashi T., Neve R., Takamiya K., and Huganir R. L. (2009) Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat. Neurosci. 12, 879–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Van Dolah D. K., Mao L. M., Shaffer C., Guo M. L., Fibuch E. E., Chu X. P., Buch S., and Wang J. Q. (2011) Reversible palmitoylation regulates surface stability of AMPA receptors in the nucleus accumbens in response to cocaine in vivo. Biol. Psychiatry 69, 1035–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Venne A. S., Kollipara L., and Zahedi R. P. (2014) The next level of complexity: crosstalk of posttranslational modifications. Proteomics 14, 513–524 [DOI] [PubMed] [Google Scholar]
- 100.Swaney D. L., Beltrao P., Starita L., Guo A., Rush J., Fields S., Krogan N. J., and Villén J. (2013) Global analysis of phosphorylation and ubiquitylation cross-talk in protein degradation. Nat. Methods 10, 676–682 [DOI] [PMC free article] [PubMed] [Google Scholar]