The Human Microcirculation – Regulation of Flow and Beyond (original) (raw)
. Author manuscript; available in PMC: 2017 Jan 8.
Abstract
The microcirculation is responsible for orchestrating adjustments in vascular tone to match local tissue perfusion with oxygen demand. Beyond this metabolic dilation, the microvasculature plays a critical role in modulating vascular tone by endothelial release of an unusually diverse family of compounds including nitric oxide, other reactive oxygen species, and arachidonic acid metabolites. Animal models have provided excellent insight into mechanisms of vasoregulation in health and disease. However there are unique aspects of the human microcirculation that serve as the focus of this review. The concept is put forth that vasculo-parenchymal communication is multimodal, with vascular release of nitric oxide eliciting dilation and preserving normal parenchymal function by inhibiting inflammation and proliferation. Likewise, in disease or stress, endothelial release of ROS both mediates dilation and parenchymal inflammation leading to cellular dysfunction, thrombosis, and fibrosis. Some pathways responsible for this stress-induced shift in mediator of vasodilation are proposed. This paradigm may help explain why microvascular dysfunction is such a powerful predictor of cardiovascular events, and help identify new approaches to treatment and prevention.
Keywords: vasodilation, oxidative stress, microvascular dysfunction, endothelial shear stress, vascular smooth muscle
Subject Terms: Endothelium/Vascular Type/Nitric Oxide, Coronary Circulation, Oxidant Stress, Coronary Artery Disease, Vascular Disease
“It has long been an axiom of mine that the little things are infinitely the most important” Sir Arthur Conan Doyle
Tasked with providing nutrients and removing metabolic byproducts from virtually all living cells in the body, the microcirculation plays a critical but indirect role in tissue function. Herein lies a major challenge. The microcirculation must maximize the distribution of nutrients and oxygen by touching nearly every living cell, while minimizing the space it occupies to allow room for the network of parenchymal cells, structural tissue, nerves, inflammatory, and other cell types that contribute more directly to organ function. The circulation comprises only about 7% of the body’s volume but is arranged to be ubiquitously distributed. This is achieved by a complex but highly organized branching pattern that can be described in terms of fractal geometry (the pattern has a fractional dimension as a result of the reiterative branching pattern)1. With diseases such as diabetes, the fractal dimension decreases, possibly contributing to less efficient tissue perfusion2, 3.
Traditionally, studies of the coronary microcirculation have focused on vasoregulation and perfusion in animal models, providing excellent insight into mechanisms of metabolic dilation, biomechanical properties of myocardial perfusion, collateral development, and endothelial regulation of coronary resistance in health and disease. Only recently has technology been sufficient to assess microcirculatory function in intact humans. As a result, it is becoming apparent that the microcirculation is importantly involved in a variety of pathological conditions and that microvascular dysfunction may herald, either as a marker and/or mechanism, the development of cardiovascular disease. This review will discuss the growing recognition that the microcirculation contributes to the development of atherosclerotic cardiovascular disease, review regulation of the coronary microcirculation in humans, and discuss potential novel functions of the microcirculation, beyond regulating perfusion that might explain the intimate link to cardiovascular disease.
Recognition of microvascular disease in humans
One of the difficulties in reading the microvascular literature is that the terms “microvascular disease” and “microvascular dysfunction” refer to a heterogeneous set of conditions ranging from reduced organ maximal perfusion, to impaired endothelium-dependent dilation of isolated arterioles. Microvascular angina is a case in point. This condition signifies “microvascular dysfunction” as a diagnosis of exclusion made in patients presenting with angina pectoris, non-obstructive coronary arteries at catheterization, and no obvious primary etiology4. The syndrome was originally thought to represent an abnormality in the coronary microcirculation since it was associated with reduced coronary flow reserve (CFR: peak to resting flow ratio)5, 6, although the definition was later expanded to include patients with normal CFR and a disturbance in pain perception7. In this review “microvascular dysfunction” refers to a reduction in maximal vasodilator capacity of the heart.
Earlier studies utilized invasive methods to assess microvascular function (catheterization-based techniques) or expensive, less invasive imaging such a positron emission tomography. Since the development of electron beam computed tomography and cardiac magnetic resonance imaging, relatively non-invasive, accessible and accurate measurements of coronary perfusion are now possible in humans. This has been instrumental in producing a body of literature identifying reduced CFR as an independent prognostic factor for adverse cardiovascular outcomes8–15. Prediction of cardiovascular events may even be greater for microvascular than conduit coronary disease although this finding is not universal16. Growing recognition that microcirculatory abnormalities contribute to cardiac prognosis supports the need for a more direct and focused understanding of how microvascular function is regulated in humans.
Lanza and colleagues 4 nicely reviewed the syndrome of primary microvascular angina, highlighting the varied nature of this diagnosis through a classification based on the clinical situation and underlying mechanisms. Microvascular angina can present as a chronic stable condition or as part of an acute coronary syndrome, both associated with microvascular pathology. Microvascular dysfunction may be manifest in the downstream branches of a specific conduit artery, or sporadically across the heart and independent of epicardial artery perfusion territory4, making detection difficult. Mild diffuse microvascular dysfunction may not reduce systolic contraction, or evoke classic ischemic ECG changes or scintographic perfusion defects, often delaying or obscuring diagnosis. Heightened suspicion and repeated diagnostic evaluation may be needed to establish microvascular dysfunction.
A number of conditions are closely associated with microvascular dysfunction. Diabetes Mellitus manifests multi-organ pathology resulting from microvascular dysfunction. Nahser et al. showed lower CFR and reduced metabolic dilation in diabetes, signs of impaired microvascular function, 17. Di Carli et al. expanded this observation by showing normal baseline flow, but similarly reduced endothelium-dependent and endothelium-independent (dilation to adenosine) microvascular dilator capacity in young subjects with either type I or type II diabetes18. Reduced dilation to adenosine persisted after controlling for duration of diabetes, insulin treatment and autonomic neuropathy18.
More direct impairment of microvascular function occurs following sustained ischemia, manifest as reduced maximal flow on CT or MRI in the absence of conduit stenosis. This phenomenon, due to microvascular blockage, is typically seen after stenting with low TIMI flow (angiographic “no reflow”) and is independently associated with major adverse cardiac events19. The cause of microvascular obstruction is multifactorial including anatomical obstruction (clumped leukocytes or platelets20, perivascular edema and/or cell swelling21) , reduced vasoreactivity, or frank microvascular necrosis. A combination of intrinsic and extrinsic obstruction may result from intramyocardial hemorrhage which occurs after prolonged ischemia in pigs22. Given that current treatment strategies emphasize early and intense anti-thrombotic treatment following percutaneous interventions, new strategies to minimize downstream microvascular hemorrhage while preventing upstream thrombosis could improve outcomes following myocardial infarction.
It is generally assumed that reperfusion plays a significant role in the magnitude of “no-reflow” and microvascular occlusion. Early and sudden reperfusion (e.g. percutaneous coronary intervention) may reduce the duration of myocardial ischemia but reintroduces blood elements and raises intravascular pressure into a distressed region of the heart with fragile, often damaged blood vessels. Staged or delayed reperfusion could obviate some of this reperfusion damage, in fact recent provocative data in humans support that idea. In a randomized prospective trial of patients presenting with ST elevation myocardial infarction, a strategy of delayed stenting/reperfusion resulted in less “no-reflow” and greater myocardial salvage23. Strategies to reduce tissue swelling and avoid leukocyte and/or platelet activation could further minimize post-ischemic microvascular and consequent cardiac damage.
Other conditions associated with microvascular dysfunction are listed in the Table. Given the wide array of diseases for which microvascular dysfunction may play a causal role, identification of biomarkers for microvascular dysfunction will be an important future clinical direction which may unveil novel risk factors for cardiovascular events and improve diagnostic and prognostic assessment.
Table.
Conditions Linked to Microvascular Dysfucntion
| Ischemic Cardiomyopathy |
|---|
| Diabetes Mellitus |
| Obstructive Sleep Apnea |
| HFpEF |
| HFrEF |
| Aging |
| Schizophrenia |
| Dementia |
| Peripheral Neuropathy |
| Chagas Disease |
| Amyloidosis |
| Chronic Thromboembolic Pulmonary Hypertension |
| Stress Related Cardiomyopathy |
| Systemic Lupus Erythematosis |
| Cerebral Vasospasm |
| Tumor Angiogenesis |
| No-Reflow Phenomenon |
| Inflammatory Bowel Disease |
| Tobacco Abuse |
| Hypertrophic Obstructive Cardiomyopathy |
| Obesity |
| Systemic Sclerosis |
| Hypertension |
| Idiopathic Cardiomyopathy |
The microcirculation as a window into conduit artery disease and cardiovascular events
Abnormal microvascular function typically precedes and predicts the development of conduit artery atherosclerosis and its risk factors. Even with minimal coronary artery disease (CAD) acetylcholine-induced vasodilation is impaired24, 25. Framingham risk score is an independent predictor of microvascular dysfunction in patients without obstructive coronary disease26. This is consistent with the idea that the microcirculation is a proverbial “canary in the mine shaft”, affected by risk factors for cardiovascular disease, evident prior to the onset of angiographic atherosclerosis26. Indeed in patients with exertional angina, no obstructive coronary disease and with normal conduit artery endothelial function, there is evidence for microvascular endothelial dysfunction27.
Women, who show a higher prevalence of angina syndromes with normal conduit arteries, have a higher degree of microvascular disease as observed in the Women Ischemia Syndrome Evaluation (WISE) trial of women undergoing evaluation for chest pain. In those with normal conduit coronary arteries, reduced CFR is present but is not associated with traditional risk factors for atherosclerosis28, 29. In these subjects a reduced CFR30 or a reduction in coronary endothelial function29 was independently predictive of future coronary events. Halcox et al. confirmed microvascular endothelial dysfunction as a risk factor for major cardiovascular events independent of coronary atherosclerosis9. We speculate that reduced microvascular dilation may predispose tissue to inflammation, facilitating the development of epicardial atherosclerosis. Impaired microvascular dilator capacity may also corrupt the ability to match tissue oxygen demand and supply. This would evoke ischemia and eventually inflammation and vascular proliferation as well as parenchymal cell dysfunction. Particularly susceptible is the heart, where oxygen supply must be linked to demand through rapidly responsive changes in flow. Impaired flow regulation could initiate a viscous inflammatory cycle culminating in myocyte damage and heart failure. Better understanding the mechanism of microvascular dysfunction should help manage conduit artery disease by reversing the pro-atherosclerotic microvascular milieu that may fuel the development of CAD and heart failure.
Microcirculatory dysfunction has been implicated in a wide variety of pathologies (Table). Microvascular dysfunction contributes to inflammation in visceral fat, which may explain the associated elevation in cardiovascular risk in subjects with excess visceral adiposity31. Microcirculatory dysfunction contributes etiologically or has a primary association with a myriad of diseases including obstructive sleep apnea32, hypertrophic cardiomyopathy10, stress-related cardiomyopathy33, congestive heart failure with reduced ejection fraction34, idiopathic cardiomyopathy35, heart failure with preserved ejection fraction36, 37, inflammatory bowel disease38, 39, schizophrenia40, amyloidosis41, tobacco use42, aging43, systemic lupus erythematosus44, and even a sedentary life style45. Abnormalities in the microcirculation are responsible for no-reflow phenomenon (reviewed by Feher et al.46), damage from cardioplegic arrest47, coronary microvascular spasm48, cerebral vasospasm following subarachnoid hemorrhage49, and angiogenesis50, 51, including tumor angiogenesis52. To the extent that these associations are confirmed as causal, the implications for treatment and prevention will be substantial. Much of the benefit of statins and angiotensin converting enzyme inhibitors may relate to previously under-considered targets in the microcirculation 53, 54. Future clinical trials to evaluate therapies designed to improve microvascular function, may also provide benefit for conduit artery disease and for cardiomyopathies associated with arteriolar dysfunction. To this end, soluble epoxide hydrolase inhibitors, being considered for the treatment of hypertension, increase epoxyeicosatrienoic acids (EET) in human coronary arterioles and can augment vasodilation via these vasoprotective compounds55. Potential benefits of enhancing EET release include reduced apoptosis56 and inhibition vascular inflammation, thrombosis, and proliferation57.
The broad array of diseases associated with microvascular dysfunction has spawned interest in identifying genetic associations. In over 600 patients with non-obstructive conduit coronary arteries, Yoshino et al. sought SNPs from 76 candidate genes. In those with low CFR (<2.5), single nucleotide polymorphisms (SNPs) within VEGFA and CDKN2B (important in vascular cell proliferation) were more prevalent. SNPs for MYH15 (codes for myosin heavy chain), VEGFA, and NT5E (associated with vascular calcification) were associated with low CFR in men but not women. In a prospective study, Fedele et al.58 identified specific polymorphisms in the gene for eNOS, and for ion channels including Kir6.2 (Katp channels), and Nav1.5 (sodium channel) in patients undergoing cardiac catheterization. SNPs in eNOS and in Kir6.2 were specific for subjects with microcirculatory dysfunction. In vasospastic angina or microvascular angina, Mashiba et al.59 found a higher frequency of SNPs in paraoxonase 1 (PON1; A632G), but not in other oxidative stress enzymes tested. Slow coronary flow is associated with a polymorphism of eNOS. Stratifying patients by presence or absence of the eNOS4a/b variant predicts low TIMI frame count on angiography in multivariate analysis with an odds ratio of 3.22 (p<0.05)60. These association studies provide impetus for directed evaluation of these pathways as mechanistically important therapeutic targets in personalized medicine.
The microcirculation can be most easily examined in the periphery including skin, retina, mucosal tissues, and limb vasculature, although these approaches are not often used clinically. Katoh and colleagues showed that the femoral blood flow increases to acetylcholine, indicative of resistance arteriolar endothelial function, correlated with risk factors for cardiovascular disease61. Endothelial dysfunction in the retinal microcirculation is also an indicator of risk for atherosclerotic cardiovascular disease 62. Even changes in the sublingual microvascular anatomy are predictive of complications in patients with acute coronary syndrome8. It is becoming evident that changes in microvascular function precede and predict conduit artery pathology. Since assessing coronary microvascular function is technically more challenging than for peripheral arterioles (see below), use of the peripheral microvasculature as a window into systemic and coronary arteriolar function would provide a paradigm shift in how we assess cardiac risk. Future studies should address whether cutaneous, retinal, or mucosal arteriolar function correlates with coronary microvascular function in the same way that brachial artery FMD serves as a viable index of coronary conduit artery endothelial function63.
Assessing human microcirculatory function
Most methods to interrogate human coronary microvascular reactivity are invasive and indirect, measuring target organ flow reserve. In the heart this is typically calculated as CFR, the ratio between maximal flow during reperfusion or pharmacological dilation, and basal flow. CFR can be assessed using intracoronary Doppler, coronary sinus thermodilution (technically challenging and less accurate) or by measuring transit time of radiopaque dyes traversing conduit coronary arteries. This latter method, codified in the Thrombolysis in Myocardial Infarction (TIMI) clinical trials as TIMI flow grade (wash-in speed of dye past a stenosis) or as the more quantifiable corrected TIMI frame count (CTFC) (number of angiographic frames it takes for the leading edge of intracoronary dye to traverse a coronary artery) is relatively easy to measure, correlates with outcomes following reperfusion, and remains widely used today64–66.
Each of these methods can quantitatively detect myocardial no-reflow or microvascular dysfunction. However, these approaches are associated with three major limitations. First, with rare exception direct visualization is not possible using non-invasive techniques. Second, information from these techniques does not differentiate dysfunction within the endothelium from that arising in the VSMC or due to extravascular compression. Third, these techniques are confounded by local neurohumoral influences, circulating substances, and paracrine or mechanical impact from underlying parenchymal cells. Moreover, most techniques require careful training and are costly, making it difficult to include measurements of microcirculatory function in large populations studies67. More recently three non-invasive methods have been validated to assess microvascular flow. These include magnetic resonance angiography, (MRA), rapid acquisition CT, and echo contrast displacement. The latter technique involves use of high energy ultrasound to destroy injected microbubbles in the myocardium and assess microvascular flow by observing the time for reopacification of the heart68. As a result of these innovations, in vivo study of the human microcirculation is now easier but confounding influences of neurohumoral compounds, metabolic dilation, and blood elements persist.
To circumvent many of these problems, vasomotor function can be studied directly in vitro using human tissue samples. This review will focus on what has been learned from direct measures of microvascular function in human tissue, including data from animal studies for comparison. This approach provides unprecedented insight into microvascular function in humans, highlighting mechanisms of disease and opportunities for treating the array of conditions where disturbances in microcirculatory function are thought to play a role (Table).
Direct videomicroscopy using cannulated, pressurized arterioles is a specialized approach for assessing microvascular function in vitro. Fresh tissue is obtained from living subjects either via biopsy or during already planned surgical procedures. Arterioles are dissected from the tissue and cannulated with micropipettes filled with physiological fluid and connected to a reservoir column to maintain an estimated physiological intraluminal pressure. The entire system is monitored with a videomicroscope and intra- and extraluminal fluids can be manipulated independently, allowing changes in vascular diameter to graded chemical or physical stimuli to be quantified directly. Because surgical acquisition of tissue is required, only a few laboratories utilize this technique in studies of human vessels. Over two decades of investigation show that human arteriolar responses are often different from those in animals and establish the importance of using human tissue for determining which animal models best recapitulate the human condition.
Role of the microcirculation in tissue homeostasis
The traditional role of the arterial microcirculation is to regulate vascular resistance and match metabolic demand with blood flow. In the heart this regulation occurs on a second-to-second basis to optimize cardiac performance and prevent ischemia. In addition to the dialog between cardiac metabolism and arteriolar tone, vascular resistance is modulated by a prominent neurohumoral influence during exercise or stress, and by myogenic tone69. Myogenic constriction protects the down-stream vasculature from damaging effects of acute elevations in pressure, prevents excessive flow to the perfused tissue and establishes capacity for flow reserve. Other modulators of vascular tone include cell-cell electrical coupling and endothelial derived factors (e.g. nitric oxide [NO], prostacyclin, endothelium-derived hyperpolarization via factors [EDHFs] , endothelin-1, thromboxane A2) that migrate to the underlying smooth muscle and elicit relaxation or contraction. Tremendous species and organ level variation exists with respect to the specific mediator and the relative impact on arteriolar tone. Even along the length of the same coronary vessel, receptor density and responses to vasomotor stimuli can vary70, 71. Conversely in response to the same stimulus, the mediator of dilation can vary across vascular beds or species. For example, arteriolar flow-mediated dilation (FMD) in the porcine coronary arteriolar bed is mediated by NO72. In rat cremaster vessels, FMD is mediated by vasodilator prostaglandins73, while in female eNOS null mice, skeletal muscle FMD is due to endothelial release of EETs74. In human, dog, and rodent ventricular arterioles, acetylcholine initiates an endothelium-dependent dilation mediated by NO. However in the porcine coronary circulation, an endothelium-independent constriction is seen75.
It is fascinating that the body utilizes such a rich cornucopia of endothelial-derived dilator substances to modulate microcirculatory tone, but the teleological importance is not clear. Further insight might be gained from recent evidence for a less traditional role of the microcirculation. Each of the endothelial mediators of vasodilation is released abluminally from arterioles where they act on vascular smooth muscle cells (VSMC) to elicit dilation, hypertrophy, or fibrosis. These mediators also penetrate to the underlying parenchymal cells especially from the capillary bed with its large surface area and lack of insulation from intervening VSMCs. Thus the vascular organ is ideally situated to exert paracrine effects on the underlying parenchymal tissue.
The microcirculation is architecturally designed for this novel form of local regulation since its intimate support of every organ necessitates close proximity to parenchymal cells. Vascular paracrine regulation of tissue function could help explain the diversity of factors produced by the endothelium (NO, prostacyclin, EETs, hydrogen peroxide [H2O2]) which elicit similar degrees of dilation but allow for a variety of local tissue responses including proliferation, fibrosis, apoptosis, and thrombosis. In this new paradigm, the microcirculation not only responds to metabolic cues from the tissue to regulate flow, but conversely the local tissue responds to factors released from the microvessels in response to mechanical and chemical stimuli. For example, nitric oxide (NO) released from endothelial cells during flow diffuses to the underlying parenchymal tissue where it exerts potent inhibition of mitochondrial metabolism, reduces production of reactive oxygen species (ROS), and inhibits inflammation76, 77. The decrease in metabolism in turn reduces cardiac production of metabolic factors that promote dilation. This paracrine influence is bidirectional in that luminally released NO reduces platelet activation and adhesion molecule expression, thereby inhibiting thrombosis and vascular inflammation78, 79.
Other examples of local paracrine regulation by the endothelium exist. Inhibition of eNOS impairs diastolic cardiac function and promotes hypertrophy80, while in pathological situations uncoupled eNOS directly contributes to oxidative stress and associated cardiac dysfunction. During disease or stress, endothelial release of NO is reduced and often replaced by release of endothelin-1, thromboxane A2, and ROS which evoke tissue inflammation, inhibit or activate apoptosis, and modulate contractility in cardiomyocytes81.
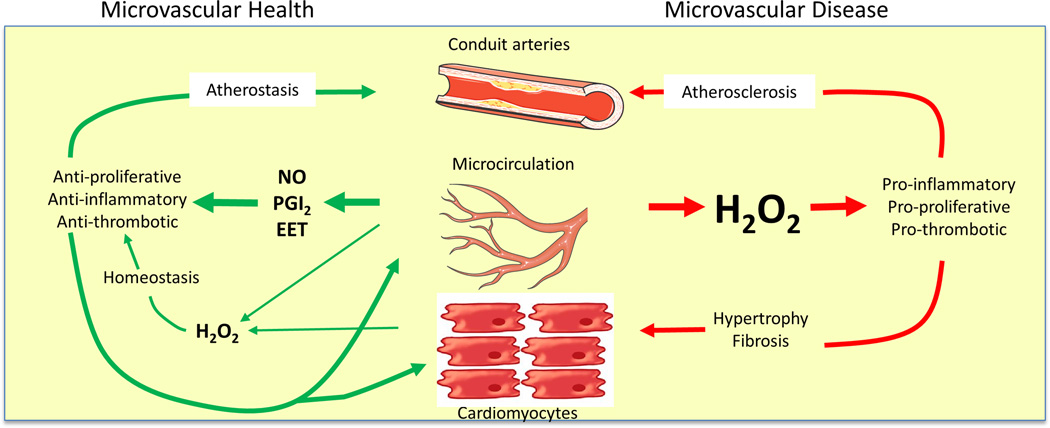
When NO bioavailability is reduced, endothelium-dependent dilation is often maintained by compensatory generation of EETs or H2O274, 82, 83. EETs are a critical intracellular component of the pathway mediating FMD in patients with CAD84, and serve as an EDHF for bradykinin-mediated dilation83. EETs inhibit vascular inflammation and apoptosis in human coronary and pulmonary endothelium56, paralleling the non-vasomotor effects of NO. In contrast H2O2, which can also compensate for loss of NO, is pro-inflammatory85–87. If released chronically from endothelial cells, H2O2 stimulates smooth muscle cell proliferation, activates endothelial cells, and promotes thrombosis. H2O2 can also modulate activity of NO and superoxide88. Thus the nature of the compensatory mediator of dilation in disease or in response to stress may not only be important for regulating vascular tone, but may also impact the health of the underlying organ (Figure 1). This effect is primarily a function of the microvasculature since conduit arteries contribute only a small fraction of an organ’s endothelial cell volume.
Figure 1.
In a healthy heart (left), arteriolar endothelium produces NO, prostacyclin, and/or epoxyeicosatrienoic acids as well as low levels of hydrogen peroxide, which support a quiescent non-proliferative state. With onset of disease, flow through the microvasculature releases hydrogen peroxide, creating a pro-inflammatory environment throughout the organ, potentially leading to hypertrophy, fibrosis, and atherosclerosis.
The concept that microvessels contribute to tissue inflammation gains traction from recent studies in adipose tissue. As reviewed by Scalia, vascular dysfunction precedes and mediates tissue inflammation in obesity31. Free fatty acids (FFA) are liberated from circulating lipid particles by lipoprotein lipase on the endothelial cell surface after a high fat meal. Since FFA are poorly taken up by endothelial cells, they initiate acute vascular inflammation in the adipose microcirculation31,89. The resulting endothelial activation promotes adipocyte inflammation, a process aggravated by obesity where the enlarged adipocyte outstrips its blood supply, fueling the hypoxic, inflammatory environment90.
Paulus and Tschope have recently proposed that microvascular dysfunction might underlie heart failure with preserved ejection fraction (HFpEF) 37. Their intriguing hypothesis posits that coronary microvascular endothelial inflammation from chronic disease (e.g. diabetes, COPD, obesity, hypertension) evokes inflammation via production of excess ROS with reduced NO bioavailability, cGMP and PKG signaling in the underling myocardium. This concept is supported by the finding of enhanced oxidative stress in endothelial cells and cardiac myocytes in biopsies of patients with HFpEF patients91. The vascular inflammation induces hyperphosphorylation of titin, promoting cardiomyocyte hypertrophy, stiffness, and fibrosis. Interestingly, the cardiotropic parvovirus B19 which selectively infects endothelial cells, is directly linked to endothelial dysfunction and HFpEF 92. This new paradigm places the microcirculation at the helm of cardiac disease development, and suggests that we refocus diagnostic, preventive, and therapeutic efforts toward understanding the microcirculatory abnormalities in subjects with heart failure with preserved ejection fraction (HFpEF), a heterogeneous group of patients who have not been responsive to conventional cardiomyopathy- targeted therapies, and for whom the need exists to more fully clarify diagnostic criteria and pathophysiology.
Beyond the autocrine and paracrine functions described, vascular cells, including the endothelium have an ingenious method for communicating with remote downstream vascular elements. Endothelial cells are capable of shedding microvesicles, small membrane-bound fragments (100nm to 1µm in diameter) that contain lipids, proteins, and microRNA93. Microvesicles provide a molecular fingerprint in terms of cell surface proteins and cytosolic contents unique to the parent cell. Once released into the vasculature, these particles bind and interact with other cell types, including downstream endothelial cells in the same tissue segment94. These couriers of biological information can then release their contents or activate surface receptors downstream. Circulating microvesicles from patients with cardiovascular disease impair endothelial function in vessels from healthy animals95. Although the majority of published studies focus on the pathological actions of microvesicles, recent reports identified particles containing micro RNAs that exert protective effects on downstream targets96. Microvesicles appear to be more than just biomarkers of vascular injury. They serve as important para/endocrine mediators in cell-to-cell communication. Given its large volume, the microcirculation probably serves as the major source of endothelial microvesicles in the circulation.
These examples define the microvasculature, the largest paracrine factory within the body, not only as a regulator of blood flow but also as a key modulator of parenchymal cell function in health and disease. It is not surprising that microcirculatory function, especially endothelial, is exquisitely important for maintaining tissue health. Using the stimulus of shear stress, ubiquitous to all endothelial cells in the body, we have begun to uncover conditions where the endothelial paracrine response changes from one that promotes tissue homeostasis (release of NO) to one that contributes to tissue inflammation and pathology (release of H2O2).
Plasticity in Microvascular Signaling
Flow mediated vasodilation refers to a process whereby blood flow activates a mechanochemical signal transduction pathway leading to smooth muscle relaxation and dilation. Present in virtually all vessels, FMD is arguably the most important physiological mechanism of endothelium-dependent vasodilation, serving to facilitate metabolic vasodilation by selectively dilating upstream vessels supplying the site of metabolic activity. A variety of endothelial derived chemical substances or direct electrical conduction to the smooth muscle can mediate FMD, depending on the tissue and species. In most animal models and in normal human tissue, NO is the principal mediator of microvascular FMD. Basal vascular diameter in the human heart is maintained by NO released from the endothelium during shear stress. Interestingly the source of this NO is neuronal nitric oxide synthase (nNOS)97, not the traditional endothelial isoform (eNOS). The relatively selective inhibitor of nNOS, S-methyl-L-thiocitrulline, reduces coronary flow but has no effect on dilation to substance P or acetylcholine, both of which were blocked by the non-specific NOS inhibitor (N(G)-monomethyl-L-arginine)97–99, thus both forms of NOS play a role in human endothelial physiology. Similar plasticity occurs in the mouse coronary circulation. Whereas wild type mice utilize eNOS for coronary dilation to flow or acetylcholine, eNOS KO mice recruit nNOS for endothelium-dependent generation of NO100, 101.
Conduit arteries including brachial and coronary vessels also demonstrate FMD. The brachial artery is an excellent index of endothelial NO production since NO is the sole mediator of FMD in that vessel. This is not the case with the downstream radial artery which in addition to NO utilizes products of cytochrome P450 2C9 epoxygenase to elicit FMD102. The magnitude of brachial FMD is inversely related to the presence of coronary disease, its risk factors, and incidence of future cardiac events103–106. Even brief insults such as transient hyperglycemia or elevations in pressure can abrogate brachial artery FMD by reducing bioavailability of NO107, 108. Brachial artery flow mediated dilation is an excellent surrogate for coronary endothelial function63 and has been used extensively in evaluating cardiovascular risk. Endothelium-dependent dilation of adipose arterioles also correlates with brachial FMD providing a link between macro and microvascular dysfunction109. Future investigation should establish whether adipose arteriolar function can serve as a surrogate for the coronary microcirculation, providing a minimally invasive window into coronary microvascular dysfunction.
In the human microcirculation, endothelium-dependent dilation is reduced in patients with CAD110 but rarely eliminated. Even in subjects with extensive CAD111, or in explanted hearts from patients with end-stage heart failure112, basal or -stimulated NO production can be observed. Brief incubation with ACE-inhibitors, statins, or sepiapterin in arterioles from subjects with significant CAD can improve endothelial dependent agonist-induced vasodilation likely by restoration of NO production113, 114. These mechanistic changes in human coronary vascular responsiveness supply critical insight regarding the best animal models of human disease. Such “reverse translation” maximizes relevance of physiological findings in animal models. To this end, Tiefenbacher et al.114 have shown that a variety of endothelium-dependent agonists stimulate uncoupled eNOS to reduce dilation in both human and pig coronary arteries. In both models an improvement in endothelial function was observed with tetrahydrobiopterin. Shimokawa’s laboratory115 has demonstrated H2O2 from uncoupled eNOS as an EDHF in the mesenteric circulation of both humans and mice.
We have examined the effect of CAD on coronary arteriolar FMD. In healthy patients, even into their seventh decade, microvascular FMD is mediated by NO116. With the onset of CAD shear stress still elicits dilation, but the mechanism changes117. Instead of NO, shear stimulates production of superoxide leading to H2O2 from proximal mitochondrial respiratory complexes 118. The mitochondrial derived H2O2 is released from the endothelial cell and taken up by the underlying VSMCs where it activates PKG1α by dimerization through oxidation of cysteine 42119. PKG1α opens large conductance calcium-activated potassium (BKCa) channels to hyperpolarize and relax the smooth muscle. The pathway of endothelial signal transduction is not fully defined but involves activation of endothelial TRPV4120 cation channels and requires an intact endothelial cytoskeleton121. EETs are a necessary component of this pathway likely via intracellular signaling84. Thus in CAD, FMD is elicited by H2O2. This contrasts with normal subjects where FMD is mediated by NO. The mechanism of this switch in dilator is important as it could impact both coronary flow regulation and tissue homeostasis. Restoration of NO as the mediator of FMD would be expected to maintain dilator capacity but also reduce inflammation, vascular proliferation, and thrombotic potential. We have recently identified two novel and critical components of the microvascular transition from health to disease that could expand therapeutic options for treating or preventing complications of CAD.
Mechanism by which the mediator of FMD changes from NO to H2O2 in the presence of CAD
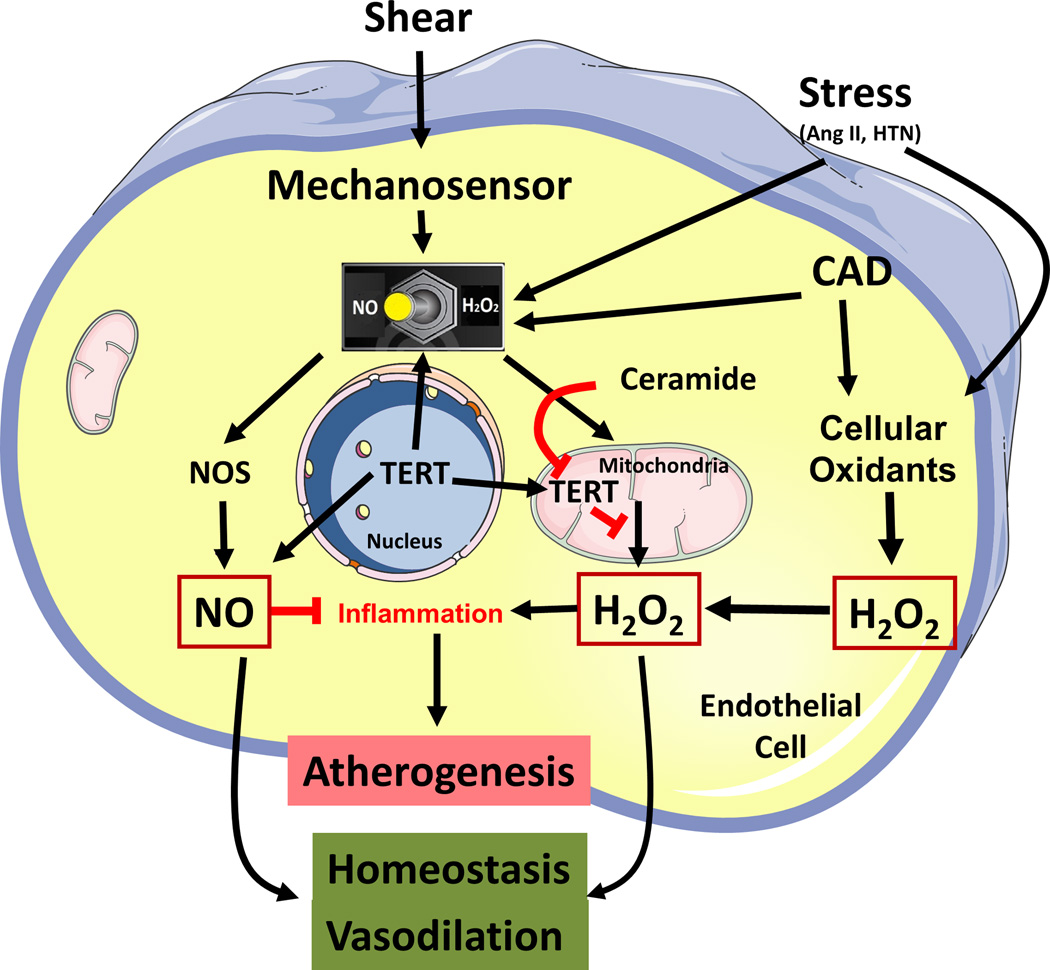
Two pathways involving production of the sphingolipid ceramide and a reduction in telomerase activity are critical in the transition from NO to H2O2 as the mediator of FMD in patients with CAD37. Ceramide, produced by neutral sphingomyelinase is a risk factor for atherosclerosis122–124, in part by stimulating ROS generation from mitochondria125, 126. Sphingomyelinase is shear-sensitive127 which strategically positions it for a role in shear-induced mitochondrial H2O2 production and therefore FMD in the diseased heart.
Treatment of vessels with ceramide overnight is sufficient to invoke a switch in the mechanism of FMD in the human heart from NO to H2O2 , and reductions in ceramide levels can restore NO as the CAD 128.These functional changes in heart microvessels were also confirmed in adipose tissue, providing generalizability across organ systems.
One of the downstream effects of ceramide is transcriptional suppression of the catalytic subunit of telomerase (TERT)129. Telomerase, typically viewed as a defender of nuclear telomere length, has been identified in the cytosol and mitochondria where it can modulate ROS production130, 131. Outside the nucleus, TERT stimulates NO production from NOS132. Mice deficient in the gene for TERT have elevated levels of mitochondrial H2O2133, 134. Thus telomerase is capable of influencing production of NO and mitochondrial ROS. Upregulation of telomerase with AGS-499 restores NO as the mediator of FMD in patients with CAD135, 136. In preliminary studies PPARγ, an inducer of telomerase, evokes the same restoration in mechanism of dilation137. Conversely inhibition of telomerase activity induces the CAD phenotype in normal human arterioles. Thus both ceramide and telomerase regulate the mediator of FMD in the human microcirculation (Figure 2). Understanding pathways of dilation and the associated mediators helps to frame options for maximizing perfusion and minimizing pathological paracrine influences in disease.
Figure 2.
Proposed mechanism for the stress-induced switch in the mediator of flow-induced dilation. In arterioles from healthy subjects, shear activates production of NO to stimulate dilation and vascular homeostasis (left side of diagram). Vascular stress or presence of coronary disease stimulates pathological basal levels of oxidants and initiates a switch in the mediator of flow-induced dilation from NO to hydrogen peroxide. This switch requires ceramide and a reduction in telomerase. Dilation is maintained but at the expense of vascular inflammation and its consequences.
Unique properties of the human microcirculation
The human coronary microcirculation exhibits several unique characteristics that have been identified through in vitro studies.
Acetylcholine mediated responses
Many vessels express muscarinic receptors on both the endothelium and smooth muscle, which when activated produce NO-mediated dilation (endothelial receptors) and/or constriction (smooth muscle receptors). Interestingly in cannulated microvessels, even if acetylcholine is administered extraluminally where it contacts the endothelium only after traversing the vascular smooth muscle, the result is dilation138. This is observed in vivo as well, where in animals parasympathetic activation elicits a coronary arteriolar vasodilation139 that is abrogated by NOS inhibition.
The coronary arteriolar response to acetylcholine is species-dependent. In the dog acetylcholine elicits vasodilation138. However the same investigators find that in the pig, only vasoconstriction is seen75. The human condition is unique in this regard. In ventricular arterioles, Ach induces an endothelium-dependent vasodilation, but in the atria, only vasoconstriction is seen 114, 140, 141. Ach is the only agonist exhibiting this differential effect in atria vs. ventricles, although human atrial vessels are capable of endothelium-dependent dilation to substance P, adenosine diphosphate, shear and bradykinin. Even a NOS-inhibitable endothelium-dependent dilation is observed in human atrial vessels to the peptide hormone adrenomedullin142, suggesting that the pathway for dilation via NOS is active. This dichotomous response to acetylcholine is observed within the same heart independent of risk factors, and occurs only in humans. Mice, rats, dogs, rabbits, cats, ferrets, and cynomolgus primates have a matched response in vessels from both atria and ventricles (unpublished observations). The pathophysiological impact of atrial constriction to Ach is not clear but may have implications for redistribution of myocardial perfusion during vagal activation or in atrial fibrillation 143.
Bradykinin-mediated human arteriolar vasodilation
Bradykinin (BK) elicits dilation via NO or EDHF in animal models. However unlike FMD there is a striking influence of gender and tissue site144. In visceral fat from premenopausal women, dilation to BK is more sensitive than in post-menopausal women or young men of similar age144. The enhanced dilator capacity is abolished by inhibiting NOS. A similar gender enhancement is observed in arterioles from subcutaneous adipose, but NO does not contribute.
Bradykinin dilation in the coronary circulation of patients with CAD is mediated by an even more unusual mechanism. As shown by Larsen et al., BK stimulates the production of H2O2 , an EDHF that opens BKCa channels in the underlying smooth muscle83, 145. This mechanism is similar to that described for shear stress but NADPH oxidase is the source of superoxide and H2O2, not mitochondria. After blocking NADPH oxidase, a residual dilation to BK is revealed. This compensatory dilation is mediated by EETs, being blocked by addition of CYP450 epoxygenase inhibitors. The EET dilation to BK is only seen in the absence of H2O2 , suggesting that H2O2 inhibits the epoxygenase. EETs emerge only as a contributing dilator when H2O2 levels are low. This was demonstrated directly using human recombinant CYP2C9 and CYP2J2146.
These observations suggest a novel hierarchical system to explain how the coronary circulation maintains the capacity for endothelium-dependent dilation with progression of disease. Under normal conditions, endothelium-dependent dilation is mediated by NO. We speculate that NO inhibits production of EETs and H2O2 under normal conditions. Early in disease with mild elevation in superoxide, NO is quenched and eNOS uncoupled, reducing NO bioavailability. In this setting, EET’s are typically produced (release of the NO-mediated inhibition of CYP450) as a compensatory dilator mechanism83, 147. With more severe oxidative stress, H2O2 blocks generation of EETs and emerges itself as a tertiary compensatory dilator, being one of the few endothelial derived dilator substances that thrives in an oxidative environment146. Additional stress may overwhelm these compensatory mechanisms or impair smooth muscle relaxation, leading to reduced dilator capacity.
Adenosine and hypoxia
Adenosine and hypoxia are potent vasodilators that participate in metabolic vasodilation. Animal studies indicate a critical role for ATP-sensitive potassium (KATP) channels in the dilation to hypoxia and adenosine(reviewed by Quayle et al.148). In humans the role of KATP channels is more complex since human coronary arteriolar dilation to adenosine is not blocked by the selective KATP channel blocker, glibenclamide149–151 while dilation to hypoxia is either inhibited152 or left unchanged149. Reasons for the difference between studies and across species are not obvious but could relate to chronicity of disease, comorbidities, or differences in the experimental preparations. The variation in response attests to the importance of confirming animal microvascular physiology in human tissue.
Microvascular response to stressors
Transient elevations in blood pressure reduce conduit artery endothelial function107 and affect the downstream microcirculation153. In both sedentary subjects and athletes, dilation of subcutaneous adipose arterioles to flow or acetylcholine is mediated by NO. Following in vitro exposure to 30 minutes of elevated intraluminal pressure FMD is reduced in sedentary subjects but maintained, albeit via H2O2 and not NO, in athletes153. A similar shift in mediator is seen using acetylcholine as the dilator agonist. It is quite unexpected that in the athlete, acute vascular stress induced by an elevation in intraluminal pressure evokes the same transition (from NO to H2O2) in dilator mechanism that occurs in patients with the chronic stress of CAD (see above).
Human gut arterioles show endothelium-dependent dilation but the mechanisms and compensatory factors during stress are unique. As shown by Matoba BK relaxes mesenteric arterioles in humans154. The principal endothelium-derived mediator is H2O2, with a smaller contribution from gap junctions154. Although the source of H2O2 was not defined, the same group found in mouse mesenteric arterioles that H2O2, derived from uncoupled NOS is a predominant EDHF155. Whether this is true in humans remains unknown, but in mucosal arterioles from patients without cardiovascular disease NO and prostacyclin mediate dilation to acetylcholine. Under the stress of inflammatory bowel disease, these vessels respond instead with a frank vasoconstriction to acetylcholine which is offset by an endothelium-independent dilation to acetylcholine via VSMC production of prostaglandin D2 (PGD2)156. The net response is a modest dilation. PGD2 plays no role in unaffected adjacent regions of bowel156; rather it is recruited specifically in the setting of inflammatory bowel disease.
Diabetes invokes a potent microvascular stress which can be partially attenuated by strict glycemic control(reviewed in157). In poorly controlled diabetic subjects, arteriolar dilation is reduced to endothelium-dependent (ADP, substance P) and independent (nitroprusside) agonists compared to non-diabetic and well-controlled subjects158. It is likely that excess ROS play a role159. Isolated human coronary arterioles from diabetic subjects with CAD demonstrate impaired dilation to hypoxia and to adenosine when compared to non-diabetic subjects152. This impairment could contribute to diabetic cardiomyopathy160. The responses to stress and disease in the human microcirculation highlight the important role of ROS as part of the signaling pathway for endothelium-dependent vasodilation. Additional dilator capacity may be summoned during stress when adrenergic tone is elevated since human coronary arteriolar endothelium expresses functionally significant amounts of beta3-adrenoreceptors161. When activated, they elicit dilation via both NO and smooth muscle hyperpolarization161.
Atrial vs. Ventricular Arteriolar Function
Much of our knowledge regarding human coronary arteriolar function derives from atrial appendage vessels obtained from discarded appendectomy specimens procured during cardiopulmonary bypass procedures. Fresh ventricular tissue is available from explanted hearts, untransplantable donor hearts, or from congenital cardiac surgery. It is therefore important to understand the extent to which atrial arterioles can serve as a surrogate for ventricular arteriolar function. Few studies have directly compared sites, however we have gained experiential data over the past 15 years indicating that with one exception (see section above “acetylcholine-mediated responses”): atrial and ventricular arterioles respond in a directionally similar manner to endothelium-dependent and independent stimuli. Importantly, in ventricular arterioles from normal subjects, NO is the mediator of FMD, but this role diminishes with CAD (unpublished observations). This finding is consistent with observations from Hintze’s laboratory showing that ventricular microvessels from diseased hearts produce less NO to Ach than vessels from non-diseased hearts162. In the future, it will be important to make better use of explanted and unused donor hearts to directly evaluate ventricular arteriolar responses. An intriguing option for acquiring viable ventricular arterioles is the rapid or “warm” autopsy procedure, used for obtaining cancer tissue within 1–4.5 hours (average 2.8) of in-hospital death163.
Role of VSMC in the regulation of human arteriolar tone
EDHFs released by the endothelium elicit dilation by hyperpolarizing underlying VSMCs which in turn blocks calcium entry via L-type Ca+2 channels, reducing vascular tone. K+ channels regulate membrane potential (Em) and therefore dilator capacity164, 165. VSMC have a membrane resistance of >108 Ohms166, thus even small changes in the electrochemical gradient can significantly alter membrane potential and vascular diameter. Vascular resistance resides mostly in the microcirculation where small changes in arteriolar diameter can result in large changes in conductance and flow as predicted by Poiseuille’s law. A 20% reduction in arteriolar diameter yields over 100% increase in resistance and reduces flow by more than 50%.
Four types of K+ channels are expressed in arteriolar smooth muscle. (1) Voltage-activated K+ channels (KV) mediate resting Em and vascular tone, elicit dilation to beta-adrenergic stimulation via cAMP167, and participate in metabolic dilation168. (2) Large conductance calcium-activated K+ channels (BKCa) maintain Em upon changes in intracellular Ca+2 levels acting as “brakes” for vasoconstrictor stimuli169, and are a primary target for EDHF55, 170 and activation by calcium sparks in human tissue171. In heightened oxidative states, Kv tend to be down-regulated while BKCa are upregulated as a compensatory influence172. (3) KATP channels respond to changes in cellular metabolism and are expressed both on the sarcolemmal membrane and on the mitochondrial outer membrane. (4) Inwardly rectifying (KIR) channels maintain the electrochemical K+ gradient and support metabolic dilation in response to small changes in extracellular potassium173. Changes in smooth muscle responsiveness in the human microcirculation are incompletely studied and most information that does exist relates to altered potassium channel responses. Our focus is on Kv and BKca since they have been studied more extensively in humans.
Kv channels
Kv channels represent a diverse group of relatively low conductance ion channels several of which (Kv1.3, Kv1.4, Kv1.5, Kv3.4, Kv4.2, Kv4.3, and Kv7) are redox sensitive174–178. Forskolin and isoproterenol open Kv channels by increasing cAMP. This dilation is impaired by high glucose179 or superoxide180, and can be restored by scavenging superoxide181. This appears to be a direct effect since single channel opening to forskolin is eliminated by incubation in high glucose179, 182. Kv channel function is also sensitive to hypercholesterolemia183, 184, and to pathophysiologically relevant concentrations of ROS in both animals and humans185. Elevated levels of ROS in CAD and its associated risk factors decrease expression of Kv channels in animals186, 187 possibly by down-regulating the transcription factor Sp1188.
The effect of disease on Kv channel function in humans is less well studied. Kv channels are redox sensitive, undergoing S-glutathionylation from physiological levels of H2O2189 with resulting dilation, but at higher cellular oxidative states, inhibition of dilation is seen190. These redox changes are particularly prominent in Kv 1.5 channels191, 192 and their modulation also occurs in the human vasculature193–195 where reversible sulfenic modification of cysteine residues are observed192. Oxidative conditions such as atrial fibrillation are associated with reduced Kv 1.5 expression194, 195. The same Kv channel subtype has recently been implicated by Ohanyan et al.196 in metabolic dilation, where it couples increases in oxygen consumption with myocardial perfusion in mice.
BKCa channels
BKCa channels are activated by both Ca2+ and membrane depolarization197, 198. These channels do not contribute to resting myogenic tone199, 200, however, they are activated by a number of endothelium-derived relaxing factors, such as NO201 and arachidonic acid metabolites202–204. Although not yet confirmed in humans, BKCa channels can be activated by calcium sparks generated from mitochondrial release of H2O2 near the sarcolemmal membrane205. These channels have been extensively studied in animals where disease tends to upregulate channel expression and function, possibly as a regulatory brake on enhanced calcium entry. For example in hypertension an increase in calcium entry through voltage-dependent calcium channels206 raises myogenic tone which is countered by upregulation in expression of the α-subunit (pore forming) of BKCa channels in the aorta207 and cerebral microvasculature208 of spontaneously hypertensive rats (SHR). This limits calcium entry by hyperpolarizing the smooth muscle cell membrane, closing L-type calcium channels and attenuating vasoconstriction.
In humans, BKCa smooth muscle activity is increased with disease. Wiecha et al. showed significantly higher activity of BKCa channels in human smooth muscle cells obtained from coronary atherosclerotic plaques compared to adjacent uninvolved arterial segments209. This is consistent with a compensatory role for BKCa in some disease states (atherosclerosis and hypertension). In other pathological conditions, BKCa channel activity is reduced. Exposure of vessels to high glucose decreases BKCa activity due to H2O2210. This effect is mediated by oxidation of a key cysteine residue in the bowl region of the channel211. Other oxidants, such as peroxynitrite, impair activity of BKCa channels in VSMC of human coronary arterioles212. However the situation is more complex in that the same oxidants (H2O2) can also enhance activity of BKCa channels in human VSMC as described above. It is unclear the circumstances that dictate which action of H2O2 is observed. This speaks to the importance of H2O2 as a versatile, highly regulated signaling molecule in the circulation.
BKCa channels are an important final target of the pathway mediating endothelium-dependent dilation in the microcirculation. Several EDHFs including EETs and H2O2 activate BKCa in the underlying smooth muscle213. NO mediated vasodilation involves phosphorylation of PKG1α which opens BKCa channels214. Disease can modify BKCa-related vasomotion either directly by modifying expression or sensitivity of K channels, or indirectly by impeding the action of endothelial dilator substances that operate through activation of K-channels. We speculate that the intracellular site of H2O2 formation, as well as the amount produced and local antioxidant levels, determine the net effect on channel activity and vascular tone.
BKCa channels and Kv channels work together to mediate dilation to H2O2. Preliminary data from the Zhang lab show that in patients without CAD, H2O2 dilation depends upon opening of Kv and BKCa channels215. However in patients with CAD, only BKCa channels contribute to the response. This could explain the slight attenuation in maximal dilation that occurs in patients with CAD where Kv no longer play a role. It also highlights the complexities of redox regulation of microvascular function where cardiovascular stress through oxidative mechanisms not only changes the mediator of endothelium-dependent dilation (NO to H2O2) but also affects the VSMC potassium channels that respond to H2O2. Depending on the amount of ROS produced and the regional intracellular concentration, the net effect can be either impaired dilation or frank constriction.
Conclusion
The human microcirculation which plays a critical role in regulating tissue perfusion, is becoming increasingly recognized as a paracrine modulator of the local tissue environment. This provides a dual role for the multitude of dilator and constrictor factors released from the endothelium that also influence downstream vessels, and parenchymal cell function. Cardiovascular stress and disease expose the dynamic nature of these vascular derived mediators which can be changed either acutely (changes in intraluminal pressure) or chronically (presence of CAD). We have reviewed some of the putative mechanisms by which these changes in dilator pathways occur, providing insight for reversal of the microcirculation-induced pro-atherosclerotic environment associated with chronic disease. A better understanding of these pathways is needed, since corruption of signaling within the microcirculation is now recognized as an etiological factor for a growing number of diseases.
Acknowledgement
The authors appreciate the critical review provided by Michael Widlansky, MD.
Sources of funding
This review was supported by funding from NHLBI, American Heart Association, and the Northwestern Mutual Chair in Cardiology.
Nonstandard Abbreviations and Acronyms
ACE
Angiotensin Converting Enzyme
Ach
Acetylcholine
ADP
Adenosine Diphosphate
ATP
Adenosine Triphosphate
BK
Bradykinin
BKca Channel
Large Conductance Calcium-Activated Potassium Channel
CAD
Coronary Artery Disease
cAMP
Cyclic Adenosine Monophosphate
CDKN2B
Cyclin-Dependent Kinase Inhibitor 2B
CFR
Coronary Flow Reserve
cGMP
Cyclic Guanosine Monophosphate
COPD
Chronic Obstructive Pulmonary Disease
CT
Computerized Tomography
CTFC
Corrected TIMI Frame Count
CVD
Cardiovascular Disease
CYP2C9
Cytochrome P450 2C9
CYP2J2
Cytochrome P450 2J2
CYP450
Cytochrome P450
ECG
Electrocardiogram
EDHF
Endothelial Derived Hyperpolarizing Factor
EET
Epoxyeicosatrienoic Acids
Em
Membrane Potential
eNOS
Endothelial Nitric Oxide Synthase
FFA
Free Fatty Acid
FMD
Flow Mediated Dilation
H2O2
Hydrogen Peroxide
HFpEF
Heart Failure with Preserved Ejection Fraction
HFrEF
Heart Failure with Reduced Ejection Fraction
KATP Channel
ATP Sensitive Potassium Channel
Kir
Inwardly-Rectifying Potassium Channel
KO
Knockout
Kv Channel
Voltage Sensitive Potassium Channel
MRA
Magnetic Resonance Angiography
MRI
Magnetic Resonance Imaging
MYH15
Myosin Heavy Chain 15
NADPH
Nicotinamide Adenine Dinucleotide Phosphate
Nav1.5
Cardiac Sodium Channel 1.5
nNOS
Neuronal Nitric Oxide Synthase
NO
Nitric Oxide
NSmase
Neutral Sphingomyelinase
NT5E
Ecto-5’-Nucleotidase
PGD2
Prostaglandin D2
PKG
Protein Kinase G
PON1
Paraoxonase 1
PPARγ
Peroxisome Proliferator-Activated Receptor γ
ROS
Reactive Oxygen Species
RNA
Ribonucleic Acid
SHR
Spontaneously Hypertensive Rat
SNP
Single Nucleotide Polymorphism
TERT
Catalytic Subunit of Telomerase
TIMI
Thrombolysis in Myocardial Infarction Study
TRPV4
Transient Receptor Potential Cation Channel, Subfamily Vanilloid, Member 4
VEGFA
Vascular Endothelial Growth Factor A
VSMC
Vascular Smooth Muscle Cell
WISE
Women Ischemia Syndrome Evaluation
Footnotes
References
- 1.Bassingthwaighte JB, King RB, Roger SA. Fractal nature of regional myocardial blood flow heterogeneity. CircRes. 1989;65:578–590. doi: 10.1161/01.res.65.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avakian A, Kalina RE, Sage EH, Rambhia AH, Elliott KE, Chuang EL, Clark JI, Hwang JN, Parsons-Wingerter P. Fractal analysis of region-based vascular change in the normal and non-proliferative diabetic retina. Curr Eye Res. 2002;24:274–280. doi: 10.1076/ceyr.24.4.274.8411. [DOI] [PubMed] [Google Scholar]
- 3.Nagaoka T, Yoshida A. Relationship between retinal fractal dimensions and retinal circulation in patients with type 2 diabetes mellitus. Curr Eye Res. 2013;38:1148–1152. doi: 10.3109/02713683.2013.805232. [DOI] [PubMed] [Google Scholar]
- 4.Lanza GA, Crea F. Primary coronary microvascular dysfunction: clinical presentation, pathophysiology, and management. Circulation. 2010;121:2317–2325. doi: 10.1161/CIRCULATIONAHA.109.900191. [DOI] [PubMed] [Google Scholar]
- 5.Brush JE, Jr, Cannon RO, 3rd, Schenke WH, Bonow RO, Leon MB, Maron BJ, Epstein SE. Angina due to coronary microvascular disease in hypertensive patients without left ventricular hypertrophy. N Engl J Med. 1988;319:1302–1307. doi: 10.1056/NEJM198811173192002. [DOI] [PubMed] [Google Scholar]
- 6.Cannon RO, III, Epstein SE. “Microvascular angina” as a cause of chest pain with angiographically normal coronary arteries. AmJCardiol. 1988;61:1338–1343. doi: 10.1016/0002-9149(88)91180-0. [DOI] [PubMed] [Google Scholar]
- 7.Cannon RO, III, Camici PG, Epstein SE. Pathophysiological dilemma of syndrome x. Circ. 1992;85:883–892. doi: 10.1161/01.cir.85.3.883. [DOI] [PubMed] [Google Scholar]
- 8.den Uil CA, Lagrand WK, van der Ent M, Jewbali LS, Cheng JM, Spronk PE, Simoons ML. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2010;31:3032–3039. doi: 10.1093/eurheartj/ehq324. [DOI] [PubMed] [Google Scholar]
- 9.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 10.Cecchi F, Olivotto I, Gistri R, Lorenzoni R, Chiriatti G, Camici PG. Coronary microvascular dysfunction and prognosis in hypertrophic cardiomyopathy. NEnglJMed. 2003;349:1027–1035. doi: 10.1056/NEJMoa025050. [DOI] [PubMed] [Google Scholar]
- 11.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: the Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 12.van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ. Physiological basis and long-term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circulation Cardiovascular interventions. 2014;7:301–311. doi: 10.1161/CIRCINTERVENTIONS.113.001049. [DOI] [PubMed] [Google Scholar]
- 13.Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation. 2015;131:528–535. doi: 10.1161/CIRCULATIONAHA.114.009716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation. 2014;129:2518–2527. doi: 10.1161/CIRCULATIONAHA.113.008507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Kranenburg M, Magro M, Thiele H, de Waha S, Eitel I, Cochet A, Cottin Y, Atar D, Buser P, Wu E, Lee D, Bodi V, Klug G, Metzler B, Delewi R, Bernhardt P, Rottbauer W, Boersma E, Zijlstra F, van Geuns RJ. Prognostic value of microvascular obstruction and infarct size, as measured by CMR in STEMI patients. JACC Cardiovascular imaging. 2014;7:930–939. doi: 10.1016/j.jcmg.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 16.Rizzoni D, Porteri E, De CC, Boari GE, Zani F, Miclini M, Paiardi S, Tiberio GA, Giulini SM, Muiesan ML, Castellano M, Rosei EA. Lack of prognostic role of endothelial dysfunction in subcutaneous small resistance arteries of hypertensive patients. JHypertens. 2006;24:867–873. doi: 10.1097/01.hjh.0000222756.76982.53. [DOI] [PubMed] [Google Scholar]
- 17.Nahser PJ, Jr, Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation. 1995;91:635–640. doi: 10.1161/01.cir.91.3.635. [DOI] [PubMed] [Google Scholar]
- 18.Di Carli MF, Janisse J, Grunberger G, Ager J. Role of chronic hyperglycemia in the pathogenesis of coronary microvascular dysfunction in diabetes. JAmCollCardiol. 2003;41:1387–1393. doi: 10.1016/s0735-1097(03)00166-9. [DOI] [PubMed] [Google Scholar]
- 19.Chan W, Stub D, Clark DJ, Ajani AE, Andrianopoulos N, Brennan AL, New G, Black A, Shaw JA, Reid CM, Dart AM, Duffy SJ Melbourne Interventional Group I. Usefulness of transient and persistent no reflow to predict adverse clinical outcomes following percutaneous coronary intervention. The American journal of cardiology. 2012;109:478–485. doi: 10.1016/j.amjcard.2011.09.037. [DOI] [PubMed] [Google Scholar]
- 20.Fabregat-Andres O, Cubillos A, Ferrando-Beltran M, Bochard-Villanueva B, Estornell-Erill J, Facila L, Ridocci-Soriano F, Morell S. Mean platelet volume is associated with infarct size and microvascular obstruction estimated by cardiac magnetic resonance in ST segment elevation myocardial infarction. Blood Coagul Fibrinolysis. 2013;24:424–427. doi: 10.1097/MBC.0b013e32835d9bca. [DOI] [PubMed] [Google Scholar]
- 21.Kloner RA, Ganote CE, Jennings RB. The “no-reflow” phenomenon after temporary coronary occlusion in the dog. JClinInvest. 1974;54:1496–1508. doi: 10.1172/JCI107898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robbers LF, Eerenberg ES, Teunissen PF, Jansen MF, Hollander MR, Horrevoets AJ, Knaapen P, Nijveldt R, Heymans MW, Levi MM, van Rossum AC, Niessen HW, Marcu CB, Beek AM, van Royen N. Magnetic resonance imaging-defined areas of microvascular obstruction after acute myocardial infarction represent microvascular destruction and haemorrhage. Eur Heart J. 2013;34:2346–2353. doi: 10.1093/eurheartj/eht100. [DOI] [PubMed] [Google Scholar]
- 23.Carrick D, Oldroyd KG, McEntegart M, Haig C, Petrie MC, Eteiba H, Hood S, Owens C, Watkins S, Layland J, Lindsay M, Peat E, Rae A, Behan M, Sood A, Hillis WS, Mordi I, Mahrous A, Ahmed N, Wilson R, Lasalle L, Genereux P, Ford I, Berry C. A randomized trial of deferred stenting versus immediate stenting to prevent no- or slow-reflow in acute ST-segment elevation myocardial infarction (DEFER-STEMI) J Am Coll Cardiol. 2014;63:2088–2098. doi: 10.1016/j.jacc.2014.02.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeiher AM, Drexler H, Wollschlager H, Just H. Endothelial dysfunction of the coronary microvasculature is associated with coronary blood flow regulation in patients with early atherosclerosis. Circulation. 1991;84:1984–1992. doi: 10.1161/01.cir.84.5.1984. [DOI] [PubMed] [Google Scholar]
- 25.Gilligan DM, Guetta V, Panaza JA, Garcia CE, Quyyumi AA, Cannon RO, III, Badar DA, Kilcoyne CM. Selective loss of microvascular endothelial function in human hypercholesterolemia. Circ. 1994;90:35–41. doi: 10.1161/01.cir.90.1.35. [DOI] [PubMed] [Google Scholar]
- 26.Rubinshtein R, Yang EH, Rihal CS, Prasad A, Lennon RJ, Best PJ, Lerman LO, Lerman A. Coronary microcirculatory vasodilator function in relation to risk factors among patients without obstructive coronary disease and low to intermediate Framingham score. EurHeart J. 2010;31:936–942. doi: 10.1093/eurheartj/ehp459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeiher AM, Krause T, Schashinger V, Minners J, Moser E. Impaired endothelium-dependent vasodilation of coronary resistance vessels is associated with exercise-induced myocardial ischemia. Circ. 1995;91:2345–2352. doi: 10.1161/01.cir.91.9.2345. [DOI] [PubMed] [Google Scholar]
- 28.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. AmHeart J. 2001;141:735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- 29.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA, National Heart L, Blood I. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation (WISE) Circulation. 2004;109:722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- 30.Pepine CJ, Anderson D, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Merz NB. Coronary Microvascular Reactivity to Adenosine Predicts Adverse Outcome in Women Evaluated for Suspected Ischemia Results From the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) Study. Journal of the American College of Cardiology. 2010;55:2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scalia R. The microcirculation in adipose tissue inflammation. Reviews in endocrine & metabolic disorders. 2013;14:69–76. doi: 10.1007/s11154-013-9236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patt BT, Jarjoura D, Haddad DN, Sen CK, Roy S, Flavahan NA, Khayat RN. Endothelial Dysfunction in the Microcirculation of Patients with Obstructive Sleep Apnea. AmJRespirCrit Care Med. 2010 doi: 10.1164/rccm.201002-0162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rigo F, Sicari R, Citro R, Ossena G, Buja P, Picano E. Diffuse, marked, reversible impairment in coronary microcirculation in stress cardiomyopathy: a Doppler transthoracic echo study. Annals of medicine. 2009;41:462–470. doi: 10.1080/07853890903022793. [DOI] [PubMed] [Google Scholar]
- 34.Treasure CB, Vita JA, Cox DA, Fish RD, Gordon JB, Mudge GH, Colucci WS, Sutton MG, Selwyn AP, Alexander RW. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation. 1990;81:772–779. doi: 10.1161/01.cir.81.3.772. [DOI] [PubMed] [Google Scholar]
- 35.Laguens R, Alvarez P, Vigliano C, Cabeza Meckert P, Favaloro L, Diez M, Favaloro R. Coronary microcirculation remodeling in patients with idiopathic dilated cardiomyopathy. Cardiology. 2011;119:191–196. doi: 10.1159/000331440. [DOI] [PubMed] [Google Scholar]
- 36.Prasad A, Higano ST, Al SJ, Holmes DR, Jr, Mathew V, Pumper G, Lennon RJ, Lerman A. Abnormal coronary microvascular endothelial function in humans with asymptomatic left ventricular dysfunction. AmHeart J. 2003;146:549–554. doi: 10.1016/S0002-8703(03)00364-8. [DOI] [PubMed] [Google Scholar]
- 37.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62:263–271. doi: 10.1016/j.jacc.2013.02.092. [DOI] [PubMed] [Google Scholar]
- 38.Deban L, Correale C, Vetrano S, Malesci A, Danese S. Multiple pathogenic roles of microvasculature in inflammatory bowel disease: a Jack of all trades. AmJPathol. 2008;172:1457–1466. doi: 10.2353/ajpath.2008.070593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hatoum OA, Gauthier KM, Binion DG, Miura H, Telford G, Otterson MF, Campbell WB, Gutterman DD. Novel mechanism of vasodilation in inflammatory bowel disease. ArteriosclerThrombVascBiol. 2005;25:2355–2361. doi: 10.1161/01.ATV.0000184757.50141.8d. [DOI] [PubMed] [Google Scholar]
- 40.Moises HW, Wollschlager D, Binder H. Functional genomics indicate that schizophrenia may be an adult vascular-ischemic disorder. Transl Psychiatry. 2015;5:e616. doi: 10.1038/tp.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Migrino RQ, Hari P, Gutterman DD, Bright M, Truran S, Schlundt B, Phillips SA. Systemic and microvascular oxidative stress induced by light chain amyloidosis. IntJCardiol. 2010;145:67–68. doi: 10.1016/j.ijcard.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miura H, Toyama K, Pratt PF, Gutterman DD. Cigarette smoking impairs Na+-K+-ATPase activity in the human coronary microcirculation. AmJPhysiol Heart CircPhysiol. 2011;300:H109–H117. doi: 10.1152/ajpheart.00237.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Holowatz LA. Human cutaneous microvascular ageing: potential insights into underlying physiological mechanisms of endothelial function and dysfunction. JPhysiol. 2008;586:3301. doi: 10.1113/jphysiol.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright SA, O’Prey FM, Rea DJ, Plumb RD, Gamble AJ, Leahey WJ, Devine AB, McGivern RC, Johnston DG, Finch MB, Bell AL, McVeigh GE. Microcirculatory hemodynamics and endothelial dysfunction in systemic lupus erythematosus. ArteriosclerThrombVascBiol. 2006;26:2281–2287. doi: 10.1161/01.ATV.0000238351.82900.7f. [DOI] [PubMed] [Google Scholar]
- 45.Thijssen DH, Green DJ, Hopman MT. Blood vessel remodeling and physical inactivity in humans. J Appl Physiol (1985) 2011;111:1836–1845. doi: 10.1152/japplphysiol.00394.2011. [DOI] [PubMed] [Google Scholar]
- 46.Feher A, Chen SY, Bagi Z, Arora V. Prevention and treatment of no-reflow phenomenon by targeting the coronary microcirculation. Reviews in cardiovascular medicine. 2014;15:38–51. doi: 10.3909/ricm0699. [DOI] [PubMed] [Google Scholar]
- 47.Feng J, Liu Y, Clements RT, Sodha NR, Khabbaz KR, Senthilnathan V, Nishimura KK, Alper SL, Sellke FW. Calcium-activated potassium channels contribute to human coronary microvascular dysfunction after cardioplegic arrest. Circulation. 2008;118:S46–S51. doi: 10.1161/CIRCULATIONAHA.107.755827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohri M, Shimokawa H, Hirakawa Y, Masumoto A, Takeshita A. Rho-kinase inhibition with intracoronary fasudil prevents myocardial ischemia in patients with coronary microvascular spasm. JAmCollCardiol. 2003;41:15–19. doi: 10.1016/s0735-1097(02)02632-3. [DOI] [PubMed] [Google Scholar]
- 49.Naraoka M, Matsuda N, Shimamura N, Asano K, Ohkuma H. The role of arterioles and the microcirculation in the development of vasospasm after aneurysmal SAH. BioMed research international. 2014;2014:253746. doi: 10.1155/2014/253746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Medhora M, Daniels J, Mundey K, Fisslthaler B, Busse R, Jacobs ER, Harder DR. Epoxygenase-driven angiogenesis in human lung microvascular endothelial cells. AmJPhysiol Heart CircPhysiol. 2003;284:H215–H224. doi: 10.1152/ajpheart.01118.2001. [DOI] [PubMed] [Google Scholar]
- 51.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. ArteriosclerThrombVascBiol. 2007;27:2319–2324. doi: 10.1161/ATVBAHA.107.149450. [DOI] [PubMed] [Google Scholar]
- 52.Grgic I, Eichler I, Heinau P, Si H, Brakemeier S, Hoyer J, Kohler R. Selective blockade of the intermediate-conductance Ca2+-activated K+ channel suppresses proliferation of microvascular and macrovascular endothelial cells and angiogenesis in vivo. ArteriosclerThrombVascBiol. 2005;25:704–709. doi: 10.1161/01.ATV.0000156399.12787.5c. [DOI] [PubMed] [Google Scholar]
- 53.Durand MJ, Phillips SA, Widlansky ME, Otterson MF, Gutterman DD. The vascular renin-angiotensin system contributes to blunted vasodilation induced by transient high pressure in human adipose microvessels. American journal of physiology Heart and circulatory physiology. 2014;307:H25–H32. doi: 10.1152/ajpheart.00055.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Paraskevaidis IA, Iliodromitis EK, Ikonomidis I, Rallidis L, Hamodraka E, Parissis J, Andoniadis A, Tzortzis S, Anastasiou-Nana M. The effect of acute administration of statins on coronary microcirculation during the pre-revascularization period in patients with myocardial infraction. Atherosclerosis. 2012;223:184–189. doi: 10.1016/j.atherosclerosis.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 55.Larsen BT, Miura H, Hatoum OA, Campbell WB, Hammock BD, Zeldin DC, Falck JR, Gutterman DD. Epoxyeicosatrienoic and dihydroxyeicosatrienoic acids dilate human coronary arterioles via BK(Ca) channels: implications for soluble epoxide hydrolase inhibition. AmJPhysiol Heart CircPhysiol. 2006;290:H491–H499. doi: 10.1152/ajpheart.00927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhanasekaran A, Al-Saghir R, Lopez B, Zhu D, Gutterman DD, Jacobs ER, Medhora M. Protective effects of epoxyeicosatrienoic acids on human endothelial cells from the pulmonary and coronary vasculature. AmJPhysiol Heart CircPhysiol. 2006;291:H517–H531. doi: 10.1152/ajpheart.00953.2005. [DOI] [PubMed] [Google Scholar]
- 57.Tacconelli S, Patrignani P. Inside epoxyeicosatrienoic acids and cardiovascular disease. Front Pharmacol. 2014;5:239. doi: 10.3389/fphar.2014.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fedele F, Mancone M, Chilian WM, Severino P, Canali E, Logan S, De Marchis ML, Volterrani M, Palmirotta R, Guadagni F. Role of genetic polymorphisms of ion channels in the pathophysiology of coronary microvascular dysfunction and ischemic heart disease. Basic Res Cardiol. 2013;108:387. doi: 10.1007/s00395-013-0387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mashiba J, Koike G, Kamiunten H, Ikeda M, Sunagawa K. Vasospastic angina and microvascular angina are differentially influenced by PON1 A632G polymorphism in the Japanese. Circ J. 2005;69:1466–1471. doi: 10.1253/circj.69.1466. [DOI] [PubMed] [Google Scholar]
- 60.Ekmekci A, Gungor B, Ozcan KS, Abaci N, Ilhan E, Ekmekci SS, Kemaloglu T, Osmonov D, Ustek D, Eren M. Evaluation of coronary microvascular function and nitric oxide synthase intron 4a/b polymorphism in patients with coronary slow flow. Coron Artery Dis. 2013;24:461–467. doi: 10.1097/MCA.0b013e328363258c. [DOI] [PubMed] [Google Scholar]
- 61.Katoh A, Ikeda H, Takajo Y, Haramaki N, Murohara T, Shintani S, Kanaya S, Yokoyama S, Ueno T, Honma T, Imaizumi T. Coexistence of impairment of endothelium-derived nitric oxide and platelet-derived nitric oxide in patients with coronary risk factors. Circ J. 2002;66:837–840. doi: 10.1253/circj.66.837. [DOI] [PubMed] [Google Scholar]
- 62.Al-Fiadh AH, Farouque O, Kawasaki R, Nguyen TT, Uddin N, Freeman M, Patel SK, Burrell LM, Wong TY. Retinal microvascular structure and function in patients with risk factors of atherosclerosis and coronary artery disease. Atherosclerosis. 2014;233:478–484. doi: 10.1016/j.atherosclerosis.2013.12.044. [DOI] [PubMed] [Google Scholar]
- 63.Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, deLaGrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC, Selwyn AP. Close relation of endothelial function in the human coronary and peripheral circulations. JAmCollCardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 64.Kunadian V, Harrigan C, Zorkun C, Palmer AM, Ogando KJ, Biller LH, Lord EE, Williams SP, Lew ME, Ciaglo LN, Buros JL, Marble SJ, Gibson WJ, Gibson CM. Use of the TIMI frame count in the assessment of coronary artery blood flow and microvascular function over the past 15 years. J Thromb Thrombolysis. 2009;27:316–328. doi: 10.1007/s11239-008-0220-3. [DOI] [PubMed] [Google Scholar]
- 65.Hamada S, Nishiue T, Nakamura S, Sugiura T, Kamihata H, Miyoshi H, Imuro Y, Iwasaka T. TIMI frame count immediately after primary coronary angioplasty as a predictor of functional recovery in patients with TIMI 3 reperfused acute myocardial infarction. J Am Coll Cardiol. 2001;38:666–671. doi: 10.1016/s0735-1097(01)01424-3. [DOI] [PubMed] [Google Scholar]
- 66.Gibson CM, Murphy SA, Rizzo MJ, Ryan KA, Marble SJ, McCabe CH, Cannon CP, Van de Werf F, Braunwald E. Relationship between TIMI frame count and clinical outcomes after thrombolytic administration. Thrombolysis In Myocardial Infarction (TIMI) Study Group. Circulation. 1999;99:1945–1950. doi: 10.1161/01.cir.99.15.1945. [DOI] [PubMed] [Google Scholar]
- 67.Strain WD, Adingupu DD, Shore AC. Microcirculation on a large scale: techniques, tactics and relevance of studying the microcirculation in larger population samples. Microcirculation. 2012;19:37–46. doi: 10.1111/j.1549-8719.2011.00140.x. [DOI] [PubMed] [Google Scholar]
- 68.Wei K, Lindner J. Contrast ultrasound in the assessment of patients presenting with suspected cardiac ischemia. Crit Care Med. 2007;35:S280–S289. doi: 10.1097/01.CCM.0000260678.03628.4C. [DOI] [PubMed] [Google Scholar]
- 69.Miller FJ, Jr, Dellsperger KC, Gutterman DD. Myogenic constriction of human coronary arterioles. AmJPhysiol. 1997;273:H257–H264. doi: 10.1152/ajpheart.1997.273.1.H257. [DOI] [PubMed] [Google Scholar]
- 70.Cohen MV, Kirk ES. Differential response of large and small coronary arteries to nitroglycerin and angiotensin. CircRes. 1973;18:445–453. doi: 10.1161/01.res.33.4.445. [DOI] [PubMed] [Google Scholar]
- 71.Penny WJ, Rockman HA, Long J, Bhargava V, Carrigan K, Ibriham A, Shabetai R, Ross J, Jr, Peterson KL. Heterogeneity of vasomotor response to acetylcholine along the human coronary artery. JAmCollCardiol. 1995;25:1046–1055. doi: 10.1016/0735-1097(94)00537-z. [DOI] [PubMed] [Google Scholar]
- 72.Kuo L, Davis MJ, Chilian WM. Endothelium-dependent, flow-induced dilation of isolated coronary arterioles. AmJPhysiol. 1990;259:H1063–H1070. doi: 10.1152/ajpheart.1990.259.4.H1063. [DOI] [PubMed] [Google Scholar]
- 73.Koller A, Kaley G. Prostaglandins mediate arteriolar dilation to increased blood flow velocity in skeletal muscle microcirculation. CircRes. 1990;67:529–534. doi: 10.1161/01.res.67.2.529. [DOI] [PubMed] [Google Scholar]
- 74.Huang A, Sun D, Carroll MA, Jiang H, Smith CJ, Connetta JA, Falck JR, Shesely EG, Koller A, Kaley G. EDHF mediates flow-induced dilation in skeletal muscle arterioles of female eNOS-KO mice. AmJPhysiol Heart CircPhysiol. 2001;280:H2462–H2469. doi: 10.1152/ajpheart.2001.280.6.H2462. [DOI] [PubMed] [Google Scholar]
- 75.Myers PR, Banitt PF, Guerra RJ, Jr, Harrison DG. Role of the endothelium in modulation of the acetylcholine vasoconstrictor response in porcine coronary microvessels. CardiovascRes. 1991;25:129–137. doi: 10.1093/cvr/25.2.129. [DOI] [PubMed] [Google Scholar]
- 76.Brunori M, Giuffre A, Sarti P, Stubauer G, Wilson MT. Nitric oxide and cellular respiration. Cell Mol Life Sci. 1999;56:549–557. doi: 10.1007/s000180050452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brookes PS, Kraus DW, Shiva S, Doeller JE, Barone MC, Patel RP, Lancaster JR, Jr, Darley-Usmar V. Control of mitochondrial respiration by NO*, effects of low oxygen and respiratory state. JBiolChem. 2003;278:31603–31609. doi: 10.1074/jbc.M211784200. [DOI] [PubMed] [Google Scholar]
- 78.Yao SK, Ober JC, Krishnaswami A, Ferguson JJ, Anderson HV, Golino P, Buja LM, Willerson JT. Endogenous nitric oxide protects against platelet aggregation and cyclic flow variations in stenosed and endothelium-injured arteries. Circulation. 1992;86:1302–1309. doi: 10.1161/01.cir.86.4.1302. [DOI] [PubMed] [Google Scholar]
- 79.Broeders MA, Tangelder GJ, Slaaf DW, Reneman RS, oude Egbrink MG. Endogenous nitric oxide protects against thromboembolism in venules but not in arterioles. Arteriosclerosis, thrombosis, and vascular biology. 1998;18:139–145. doi: 10.1161/01.atv.18.1.139. [DOI] [PubMed] [Google Scholar]
- 80.Noireaud J, Andriantsitohaina R. Recent insights in the paracrine modulation of cardiomyocyte contractility by cardiac endothelial cells. BioMed research international. 2014;2014:923805. doi: 10.1155/2014/923805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Namekata I, Fujiki S, Kawakami Y, Moriwaki R, Takeda K, Kawanishi T, Takahara A, Shigenobu K, Tanaka H. Intracellular mechanisms and receptor types for endothelin-1-induced positive and negative inotropy in mouse ventricular myocardium. Naunyn-Schmiedeberg’s archives of pharmacology. 2008;376:385–395. doi: 10.1007/s00210-007-0228-9. [DOI] [PubMed] [Google Scholar]
- 82.Harder DR, Campbell WB, Roman RJ. Role of cytochrome P-450 enzymes and metabolites of arachidonic acid in the control of vascular tone. Journal of Vascular Research. 1995;32:79–92. doi: 10.1159/000159080. [DOI] [PubMed] [Google Scholar]
- 83.Larsen BT, Bubolz AH, Mendoza SA, Pritchard KA, Jr, Gutterman DD. Bradykinin-Induced Dilation of Human Coronary Arterioles Requires NADPH Oxidase-Derived Reactive Oxygen Species. ArteriosclerThrombVascBiol. 2009 doi: 10.1161/ATVBAHA.108.169367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Miura H, Wachtel RE, Liu Y, Loberiza FR, Jr, Saito T, Miura M, Gutterman DD. Flow-induced dilation of human coronary arterioles: important role of Ca(2+)-activated K(+) channels. Circulation. 2001;103:1992–1998. doi: 10.1161/01.cir.103.15.1992. [DOI] [PubMed] [Google Scholar]
- 85.Ungvari Z, Orosz Z, Labinskyy N, Rivera A, Xiangmin Z, Smith K, Csiszar A. Increased mitochondrial H2O2 production promotes endothelial NF-kappaB activation in aged rat arteries. AmJPhysiol Heart CircPhysiol. 2007;293:H37–H47. doi: 10.1152/ajpheart.01346.2006. [DOI] [PubMed] [Google Scholar]
- 86.Bychkov R, Pieper K, Ried C, Milosheva M, Bychkov E, Luft FC, Haller H. Hydrogen peroxide, potassium currents, and membrane potential in human endothelial cells. Circulation. 1999;99:1719–1725. doi: 10.1161/01.cir.99.13.1719. [DOI] [PubMed] [Google Scholar]
- 87.Ody C, Junod AF. Effect of variable glutathione peroxidase activity on H2O2-related cytotoxicity in cultured aortic endothelial cells. Proceedings of the Society for Experimental Biology and Medicine Society for Experimental Biology and Medicine. 1985;180:103–111. doi: 10.3181/00379727-180-42150. [DOI] [PubMed] [Google Scholar]
- 88.Witting PK, Rayner BS, Wu BJ, Ellis NA, Stocker R. Hydrogen peroxide promotes endothelial dysfunction by stimulating multiple sources of superoxide anion radical production and decreasing nitric oxide bioavailability. Cell Physiol Biochem. 2007;20:255–268. doi: 10.1159/000107512. [DOI] [PubMed] [Google Scholar]
- 89.Talukdar S, Oh da Y, Bandyopadhyay G, Li D, Xu J, McNelis J, Lu M, Li P, Yan Q, Zhu Y, Ofrecio J, Lin M, Brenner MB, Olefsky JM. Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat Med. 2012;18:1407–1412. doi: 10.1038/nm.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Halberg N, Khan T, Trujillo ME, Wernstedt-Asterholm I, Attie AD, Sherwani S, Wang ZV, Landskroner-Eiger S, Dineen S, Magalang UJ, Brekken RA, Scherer PE. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Molecular and cellular biology. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Westermann D, Lindner D, Kasner M, Zietsch C, Savvatis K, Escher F, von Schlippenbach J, Skurk C, Steendijk P, Riad A, Poller W, Schultheiss HP, Tschope C. Cardiac inflammation contributes to changes in the extracellular matrix in patients with heart failure and normal ejection fraction. Circulation Heart failure. 2011;4:44–52. doi: 10.1161/CIRCHEARTFAILURE.109.931451. [DOI] [PubMed] [Google Scholar]
- 92.Tschope C, Bock CT, Kasner M, Noutsias M, Westermann D, Schwimmbeck PL, Pauschinger M, Poller WC, Kuhl U, Kandolf R, Schultheiss HP. High prevalence of cardiac parvovirus B19 infection in patients with isolated left ventricular diastolic dysfunction. Circulation. 2005;111:879–886. doi: 10.1161/01.CIR.0000155615.68924.B3. [DOI] [PubMed] [Google Scholar]
- 93.Hugel B, Martinez MC, Kunzelmann C, Freyssinet JM. Membrane microparticles: two sides of the coin. Physiology. 2005;20:22–27. doi: 10.1152/physiol.00029.2004. [DOI] [PubMed] [Google Scholar]
- 94.Dignat-George F, Boulanger CM. The many faces of endothelial microparticles. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:27–33. doi: 10.1161/ATVBAHA.110.218123. [DOI] [PubMed] [Google Scholar]
- 95.Boulanger CM, Scoazec A, Ebrahimian T, Henry P, Mathieu E, Tedgui A, Mallat Z. Circulating microparticles from patients with myocardial infarction cause endothelial dysfunction. Circulation. 2001;104:2649–2652. doi: 10.1161/hc4701.100516. [DOI] [PubMed] [Google Scholar]
- 96.Zernecke A, Bidzhekov K, Noels H, Shagdarsuren E, Gan L, Denecke B, Hristov M, Koppel T, Jahantigh MN, Lutgens E, Wang S, Olson EN, Schober A, Weber C. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci Signal. 2009;2:ra81. doi: 10.1126/scisignal.2000610. [DOI] [PubMed] [Google Scholar]
- 97.Seddon MD, Chowienczyk PJ, Brett SE, Casadei B, Shah AM. Neuronal nitric oxide synthase regulates basal microvascular tone in humans in vivo. Circulation. 2008;117:1991–1996. doi: 10.1161/CIRCULATIONAHA.107.744540. [DOI] [PubMed] [Google Scholar]
- 98.Melikian N, Seddon MD, Casadei B, Chowienczyk PJ, Shah AM. Neuronal nitric oxide synthase and human vascular regulation. Trends CardiovascMed. 2009;19:256–262. doi: 10.1016/j.tcm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Seddon M, Melikian N, Dworakowski R, Shabeeh H, Jiang B, Byrne J, Casadei B, Chowienczyk P, Shah AM. Effects of neuronal nitric oxide synthase on human coronary artery diameter and blood flow in vivo. Circulation. 2009;119:2656–2662. doi: 10.1161/CIRCULATIONAHA.108.822205. [DOI] [PubMed] [Google Scholar]
- 100.Lamping KG, Nuno DW, Faraci FM. Role of nNOS in dilation to acetylcholine in coronary arteries from eNOS-deficient mice. Faseb J. 1999:A1085. [Google Scholar]
- 101.Huang A, Sun D, Shesely EG, Levee EM, Koller A, Kaley G. Neuronal NOS-dependent dilation to flow in coronary arteries of male eNOS-KO mice. AmJPhysiol Heart CircPhysiol. 2002;282:H429–H436. doi: 10.1152/ajpheart.00501.2001. [DOI] [PubMed] [Google Scholar]
- 102.Fischer D, Landmesser U, Spiekermann S, Hilfiker-Kleiner D, Hospely M, Muller M, Busse R, Fleming I, Drexler H. Cytochrome P450 2C9 is involved in flow-dependent vasodilation of peripheral conduit arteries in healthy subjects and in patients with chronic heart failure. EurJHeart Fail. 2007;9:770–775. doi: 10.1016/j.ejheart.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 103.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. JAmCollCardiol. 2003;41:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 104.Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D’Ambrosio A, Montesanti R, Di SG. Impaired flow-mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–75. doi: 10.1161/01.CIR.0000151308.06673.D2. [DOI] [PubMed] [Google Scholar]
- 105.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 106.Schachinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long- term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. [DOI] [PubMed] [Google Scholar]
- 107.Jurva JW, Phillips SA, Syed AQ, Syed AY, Pitt S, Weaver A, Gutterman DD. The effect of exertional hypertension evoked by weight lifting on vascular endothelial function. JAmCollCardiol. 2006;48:588–589. doi: 10.1016/j.jacc.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Title LM, Cummings PM, Giddens K, Nassar BA. Oral glucose loading acutely attenuates endothelium-dependent vasodilation in healthy adults without diabetes: an effect prevented by vitamins C and E. J AmCollCardiol. 2000;36:2185–2191. doi: 10.1016/s0735-1097(00)00980-3. [DOI] [PubMed] [Google Scholar]
- 109.Dharmashankar K, Welsh A, Wang J, Kizhakekuttu TJ, Ying R, Gutterman DD, Widlansky ME. Nitric oxide synthase-dependent vasodilation of human subcutaneous arterioles correlates with noninvasive measurements of endothelial function. AmJHypertens. 2012;25:528–534. doi: 10.1038/ajh.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cooper A, Heagerty AM. Endothelial dysfunction in human intramyocardial small arteries in atherosclerosis and hypercholesterolemia. AmJPhysiol. 1998;275:H1482–H1488. doi: 10.1152/ajpheart.1998.275.4.H1482. [DOI] [PubMed] [Google Scholar]
- 111.Miura H, Liu Y, Gutterman DD. Human Coronary Arteriolar Dilation to Bradykinin Depends on Membrane Hyperpolarization. Circulation. 1999;99:3132–3138. doi: 10.1161/01.cir.99.24.3132. [DOI] [PubMed] [Google Scholar]
- 112.Kichuk MR, Zhang X, Oz M, Michler R, Kaley G, Nasjletti A, Hintze TH. Angiotensin-converting enzyme inhibitors promote nitric oxide production in coronary microvessels from failing explanted human hearts. AmJCardiol. 1997;80:137A–142A. doi: 10.1016/s0002-9149(97)00469-4. [DOI] [PubMed] [Google Scholar]
- 113.Tiefenbacher CP, Friedrich S, Bleeke T, Vahl C, Chen X, Niroomand F. ACE inhibitors and statins acutely improve endothelial dysfunction of human coronary arterioles. AmJPhysiol Heart CircPhysiol. 2004;286:H1425–H1432. doi: 10.1152/ajpheart.00783.2003. [DOI] [PubMed] [Google Scholar]
- 114.Tiefenbacher CP, Bleeke T, Vahl C, Amann K, Vogt A, Kubler W. Endothelial dysfunction of coronary resistance arteries is improved by tetrahydrobiopterin in atherosclerosis [In Process Citation] Circulation. 2000;102:2172–2179. doi: 10.1161/01.cir.102.18.2172. [DOI] [PubMed] [Google Scholar]
- 115.Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, Kanaide H, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. JClinInvest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zinkevich NS, Wittenburg AL, Gutterman DD. Role of Cyclooxygenase in flow-induced dilation of human coronary arterioles depends upone age. Circulation. 2010:122. [Google Scholar]
- 117.Miura H, Qiang C, Liu Y, Saito T, Miura M, Gutterman DD. Flow-Mediated Dilation of Human Coronary Arterioles Involves Production of Hydrogen Peroxide. Circulation, Supplement II. 2001;104:II-285–II-286. [Google Scholar]
- 118.Liu Y, Zhao H, Li H, Kalyanaraman B, Nicolosi AC, Gutterman DD. Mitochondrial sources of H2O2 generation play a key role in flow-mediated dilation in human coronary resistance arteries. CircRes. 2003;93:573–580. doi: 10.1161/01.RES.0000091261.19387.AE. [DOI] [PubMed] [Google Scholar]
- 119.Burgoyne JR, Oka S, Ale-Agha N, Eaton P. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. AntioxidRedoxSignal. 2013;18:1042–1052. doi: 10.1089/ars.2012.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bubolz AH, Mendoza SA, Zheng X, Zinkevich NS, Li R, Gutterman DD, Zhang DX. Activation of endothelial TRPV4 channels mediates flow-induced dilation in human coronary arterioles: role of Ca2+ entry and mitochondrial ROS signaling. AmJPhysiol Heart CircPhysiol. 2012;302:H634–H642. doi: 10.1152/ajpheart.00717.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Liu Y, Li H, Bubolz AH, Zhang DX, Gutterman DD. Endothelial cytoskeletal elements are critical for flow-mediated dilation in human coronary arterioles. MedBiolEng Comput. 2008 doi: 10.1007/s11517-008-0331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kurek K, Piotrowska DM, Wiesiolek-Kurek P, Chabowska A, Lukaszuk B, Zendzian-Piotrowska M. The role of sphingolipids in selected cardiovascular diseases. Postepy higieny i medycyny doswiadczalnej. 2013;67:1018–1026. doi: 10.5604/17322693.1068694. [DOI] [PubMed] [Google Scholar]
- 123.Li X, Becker KA, Zhang Y. Ceramide in redox signaling and cardiovascular diseases. Cell Physiol Biochem. 2010;26:41–48. doi: 10.1159/000315104. [DOI] [PubMed] [Google Scholar]
- 124.Bismuth J, Lin P, Yao Q, Chen C. Ceramide: a common pathway for atherosclerosis? Atherosclerosis. 2008;196:497–504. doi: 10.1016/j.atherosclerosis.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Deshpande SS, Qi B, Park YC, Irani K. Constitutive activation of rac1 results in mitochondrial oxidative stress and induces premature endothelial cell senescence. ArteriosclerThrombVascBiol. 2003;23:e1–e6. doi: 10.1161/01.atv.0000047869.13737.53. [DOI] [PubMed] [Google Scholar]
- 126.Corda S, Laplace C, Vicaut E, Duranteau J. Rapid reactive oxygen species production by mitochondria in endothelial cells exposed to tumor necrosis factor-alpha is mediated by ceramide. AmJRespirCell MolBiol. 2001;24:762–768. doi: 10.1165/ajrcmb.24.6.4228. [DOI] [PubMed] [Google Scholar]
- 127.Czarny M, Liu J, Oh P, Schnitzer JE. Transient mechanoactivation of neutral sphingomyelinase in caveolae to generate ceramide. JBiolChem. 2003;278:4424–4430. doi: 10.1074/jbc.M210375200. [DOI] [PubMed] [Google Scholar]
- 128.Freed JK, Beyer AM, LoGiudice JA, Hockenberry JC, Gutterman DD. Ceramide changes the mediator of flow-induced vasodilation from nitric oxide to hydrogen peroxide in the human microcirculation. Circ Res. 2014;115:525–532. doi: 10.1161/CIRCRESAHA.115.303881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wooten-Blanks LG, Song P, Senkal CE, Ogretmen B. Mechanisms of ceramide-mediated repression of the human telomerase reverse transcriptase promoter via deacetylation of Sp3 by histone deacetylase 1. Faseb J. 2007;21:3386–3397. doi: 10.1096/fj.07-8621com. [DOI] [PubMed] [Google Scholar]
- 130.Santos JH, Meyer JN, Van Houten B. Mitochondrial localization of telomerase as a determinant for hydrogen peroxide-induced mitochondrial DNA damage and apoptosis. Hum Mol Genet. 2006;15:1757–1768. doi: 10.1093/hmg/ddl098. [DOI] [PubMed] [Google Scholar]
- 131.Haendeler J, Drose S, Buchner N, Jakob S, Altschmied J, Goy C, Spyridopoulos I, Zeiher AM, Brandt U, Dimmeler S. Mitochondrial telomerase reverse transcriptase binds to and protects mitochondrial DNA and function from damage. ArteriosclerThrombVascBiol. 2009;29:929–935. doi: 10.1161/ATVBAHA.109.185546. [DOI] [PubMed] [Google Scholar]
- 132.Matsushita H, Chang E, Glassford AJ, Cooke JP, Chiu CP, Tsao PS. eNOS activity is reduced in senescent human endothelial cells: Preservation by hTERT immortalization. CircRes. 2001;89:793–798. doi: 10.1161/hh2101.098443. [DOI] [PubMed] [Google Scholar]
- 133.Sahin E, Depinho RA. Linking functional decline of telomeres, mitochondria and stem cells during ageing. Nature. 2010;464:520–528. doi: 10.1038/nature08982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sahin E, Colla S, Liesa M, Moslehi J, Muller FL, Guo M, Cooper M, Kotton D, Fabian AJ, Walkey C, Maser RS, Tonon G, Foerster F, Xiong R, Wang YA, Shukla SA, Jaskelioff M, Martin ES, Heffernan TP, Protopopov A, Ivanova E, Mahoney JE, Kost-Alimova M, Perry SR, Bronson R, Liao R, Mulligan R, Shirihai OS, Chin L, DePinho RA. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature. 2011;470:359–365. doi: 10.1038/nature09787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Santos J, Gutterman DD, Beyer AM. Mitochondrial telomerase regulates flow mediated dilation by suppressing mitochondrial derived free radical production. FASEB J. 2014;28:664.1. [Google Scholar]
- 136.Eitan E, Tichon A, Gazit A, Gitler D, Slavin S, Priel E. Novel telomerase-increasing compound in mouse brain delays the onset of amyotrophic lateral sclerosis. EMBO Mol Med. 2012;4:313–329. doi: 10.1002/emmm.201200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Beyer AM, Gutterman DD. Activation of PPAR {gamma} Converts the Mechanism of Flow-Mediated Dilation in Human Microvessels from H2O2 to NO by a Telomerase Dependent Mechanism. Circulation. 2012;126:A9240. [Google Scholar]
- 138.Myers PR, Banitt PF, Guerra R, Jr, Harrison DG. Characteristics of canine coronary resistance arteries: importance of endothelium. Am J Physiol. 1989;257:H603–H610. doi: 10.1152/ajpheart.1989.257.2.H603. [DOI] [PubMed] [Google Scholar]
- 139.Broten TP, Miyashiro JK, Moncada S, Feigl EO. Role of endothelium-derived relaxing factor in parasympathetic coronary vasodilation. Am J Physiol. 1992;262:H1579–H1584. doi: 10.1152/ajpheart.1992.262.5.H1579. [DOI] [PubMed] [Google Scholar]
- 140.Miller FJ, Dellsperger KC, Gutterman DD. Pharmacologic activation of the human coronary microcirculation in vitro: Endothelium-dependent dilation and differential responses to acetylcholine. CardiovascRes. 1998;38:744–750. doi: 10.1016/s0008-6363(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 141.Angus JA, Cocks TM, McPherson GA, Broughton A. The acetylcholine paradox: a constrictor of human small coronary arteries even in the presence of endothelium. ClinExpPharmacolPhysiol. 1991;18:33–36. doi: 10.1111/j.1440-1681.1991.tb01373.x. [DOI] [PubMed] [Google Scholar]
- 142.Terata K, Miura H, Liu Y, Loberiza F, Gutterman DD. Human coronary arteriolar dilation to adrenomedullin: role of nitric oxide and K(+) channels. AmJPhysiol Heart CircPhysiol. 2000;279:H2620–H2626. doi: 10.1152/ajpheart.2000.279.6.H2620. [DOI] [PubMed] [Google Scholar]
- 143.Sueda S, Fukuda H, Watanabe K, Ochi N, Kawada H, Hayashi Y, Uraoka T. Clinical characteristics and possible mechanism of paroxysmal atrial fibrillation induced by intracoronary injection of acetylcholine. The American journal of cardiology. 2001;88:570–573. doi: 10.1016/s0002-9149(01)01744-1. [DOI] [PubMed] [Google Scholar]
- 144.Sato A, Miura H, Liu Y, Somberg LB, Otterson MF, Demeure MJ, Schulte WJ, Eberhardt LM, Loberiza FR, Sakuma I, Gutterman DD. Effect of gender on endothelium-dependent dilation to bradykinin in human adipose microvessels. AmJPhysiol Heart CircPhysiol. 2002;283:H845–H852. doi: 10.1152/ajpheart.00160.2002. [DOI] [PubMed] [Google Scholar]
- 145.Miura H, Liu Y, Gutterman DD. Human coronary arteriolar dilation to bradykinin depends on membrane hyperpolarization. Contribution of nitric oxide and calcium-activated K+ channels. Circ. 1999 doi: 10.1161/01.cir.99.24.3132. [DOI] [PubMed] [Google Scholar]
- 146.Larsen BT, Gutterman DD, Sato A, Toyama K, Campbell WB, Zeldin DC, Manthati VL, Falck JR, Miura H. Hydrogen peroxide inhibits cytochrome p450 epoxygenases: interaction between two endothelium-derived hyperpolarizing factors. CircRes. 2008;102:59–67. doi: 10.1161/CIRCRESAHA.107.159129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ding Z, Godecke A, Schrader J. Contribution of cytochrome P450 metabolites to bradykinin-induced vasodilation in endothelial NO synthase deficient mouse hearts. BrJPharmacol. 2002;135:631–638. doi: 10.1038/sj.bjp.0704472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Quayle JM, Nelson MT, Standen NB. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. PhysiolRev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- 149.Lynch FM, Austin C, Heagerty AM, Izzard AS. Adenosine- and hypoxia-induced dilation of human coronary resistance arteries: evidence against the involvement of K(ATP) channels. BrJPharmacol. 2006;147:455–458. doi: 10.1038/sj.bjp.0706622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kemp BK, Cocks TM. Adenosine mediates relaxation of human small resistance-like coronary arteries via A2B receptors. BrJPharmacol. 1999;126:1796–1800. doi: 10.1038/sj.bjp.0702462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sato A, Terata K, Miura H, Toyama K, Loberiza FR, Jr, Hatoum OA, Saito T, Sakuma I, Gutterman DD. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. AmJPhysiol Heart CircPhysiol. 2005;288:H1633–H1640. doi: 10.1152/ajpheart.00575.2004. [DOI] [PubMed] [Google Scholar]
- 152.Miura H, Wachtel RE, Loberiza FR, Jr, Saito T, Miura M, Nicolosi AC, Gutterman DD. Diabetes mellitus impairs vasodilation to hypoxia in human coronary arterioles: reduced activity of ATP-sensitive potassium channels. CircRes. 2003;92:151–158. doi: 10.1161/01.res.0000052671.53256.49. [DOI] [PubMed] [Google Scholar]
- 153.Beyer AM, Durand MJ, Hockenberry J, Gamblin TC, Phillips SA, Gutterman DD. An acute rise in intraluminal pressure shifts the mediator of flow-mediated dilation from nitric oxide to hydrogen peroxide in human arterioles. American journal of physiology Heart and circulatory physiology. 2014;307:H1587–H1593. doi: 10.1152/ajpheart.00557.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, Mukai Y, Hirakawa Y, Takeshita A. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. BiochemBiophysResCommun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- 155.Morikawa K, Shimokawa H, Matoba T, Kubota H, Akaike T, Talukder MA, Hatanaka M, Fujiki T, Maeda H, Takahashi S, Takeshita A. Pivotal role of Cu,Zn-superoxide dismutase in endothelium-dependent hyperpolarization. JClinInvest. 2003;112:1871–1879. doi: 10.1172/JCI19351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hatoum OA, Binion DG, Otterson MF, Gutterman DD. Acquired microvascular dysfunction in inflammatory bowel disease: Loss of nitric oxide-mediated vasodilation. Gastroenterology. 2003;125:58–69. doi: 10.1016/s0016-5085(03)00699-1. [DOI] [PubMed] [Google Scholar]
- 157.Singh A, Donnino R, Weintraub H, Schwartzbard A. Effect of strict glycemic control in patients with diabetes mellitus on frequency of macrovascular events. The American journal of cardiology. 2013;112:1033–1038. doi: 10.1016/j.amjcard.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 158.Feng J, Liu Y, Chu LM, Singh AK, Dobrilovic N, Fingleton JG, Clements RT, Bianchi C, Sellke FW. Changes in microvascular reactivity after cardiopulmonary bypass in patients with poorly controlled versus controlled diabetes. Circulation. 2012;126:S73–S80. doi: 10.1161/CIRCULATIONAHA.111.084590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shida T, Nozawa T, Sobajima M, Ihori H, Matsuki A, Inoue H. Fluvastatin-induced reduction of oxidative stress ameliorates diabetic cardiomyopathy in association with improving coronary microvasculature. Heart Vessels. 2014;29:532–541. doi: 10.1007/s00380-013-0402-6. [DOI] [PubMed] [Google Scholar]
- 160.Finck BN, Han X, Courtois M, Aimond F, Nerbonne JM, Kovacs A, Gross RW, Kelly DP. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–1231. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dessy C, Moniotte S, Ghisdal P, Havaux X, Noirhomme P, Balligand JL. Endothelial beta3-adrenoceptors mediate vasorelaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarization. Circulation. 2004;110:948–954. doi: 10.1161/01.CIR.0000139331.85766.AF. [DOI] [PubMed] [Google Scholar]
- 162.Kichuk MR, Seyedi N, Zhang XP, Marboe CC, Michler RE, Addonizio LJ, Kaley G, Nasjletti A, Hintze TH. Regulation of nitric oxide production in human coronary microvessels and the contribution of local kinin formation. Circ. 1996;94:44–51. doi: 10.1161/01.cir.94.1.44. [DOI] [PubMed] [Google Scholar]
- 163.Rubin MA, Putzi M, Mucci N, Smith DC, Wojno K, Korenchuk S, Pienta KJ. Rapid (“warm”) autopsy study for procurement of metastatic prostate cancer. Clin Cancer Res. 2000;6:1038–1045. [PubMed] [Google Scholar]
- 164.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. The American journal of physiology. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 165.Ko EA, Han J, Jung ID, Park WS. Physiological roles of K+ channels in vascular smooth muscle cells. Journal of smooth muscle research = Nihon Heikatsukin Gakkai kikanshi. 2008;44:65–81. doi: 10.1540/jsmr.44.65. [DOI] [PubMed] [Google Scholar]
- 166.Wilde DW, Lee KS. Outward potassium currents in freshly isolated smooth muscle cell of dog coronary arteries. Circulation research. 1989;65:1718–1734. doi: 10.1161/01.res.65.6.1718. [DOI] [PubMed] [Google Scholar]
- 167.Aiello EA, Clement-Chomienne O, Sontag DP, Walsh MP, Cole WC. Protein kinase C inhibits delayed rectifier K+ current in rabbit vascular smooth muscle cells. American Journal of Physiology. 1996;271:H109–H119. doi: 10.1152/ajpheart.1996.271.1.H109. [DOI] [PubMed] [Google Scholar]
- 168.Rogers PA, Dick GM, Knudson JD, Focardi M, Bratz IN, Swafford AN, Jr, Saitoh S, Tune JD, Chilian WM. H2O2-induced redox-sensitive coronary vasodilation is mediated by 4-aminopyridine-sensitive K+ channels. AmJPhysiol Heart CircPhysiol. 2006;291:H2473–H2482. doi: 10.1152/ajpheart.00172.2006. [DOI] [PubMed] [Google Scholar]
- 169.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–535. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 170.Archer SL, Gragasin FS, Wu X, Wang S, McMurtry S, Kim DH, Platonov M, Koshal A, Hashimoto K, Campbell WB, Falck JR, Michelakis ED. Endothelium-derived hyperpolarizing factor in human internal mammary artery is 11,12-epoxyeicosatrienoic acid and causes relaxation by activating smooth muscle BK(Ca) channels. Circulation. 2003;107:769–776. doi: 10.1161/01.cir.0000047278.28407.c2. [DOI] [PubMed] [Google Scholar]
- 171.Furstenau M, Lohn M, Ried C, Luft FC, Haller H, Gollasch M. Calcium sparks in human coronary artery smooth muscle cells resolved by confocal imaging. JHypertens. 2000;18:1215–1222. doi: 10.1097/00004872-200018090-00007. [DOI] [PubMed] [Google Scholar]
- 172.Li Z, Lu N, Shi L. Exercise training reverses alterations in Kv and BKCa channel molecular expression in thoracic aorta smooth muscle cells from spontaneously hypertensive rats. J Vasc Res. 2014;51:447–457. doi: 10.1159/000369928. [DOI] [PubMed] [Google Scholar]
- 173.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12:113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Stephens GJ, Owen DG, Robertson B. Cysteine-modifying reagents alter the gating of the rat cloned potassium channel Kv1.4. Pflugers Arch. 1996;431:435–442. doi: 10.1007/BF02207283. [DOI] [PubMed] [Google Scholar]
- 175.Duprat F, Guillemare E, Romey G, Fink M, Lesage F, Lazdunski M, Honore E. Susceptibility of cloned K+ channels to reactive oxygen species. Proc Natl Acad Sci U S A. 1995;92:11796–11800. doi: 10.1073/pnas.92.25.11796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Li X, Tang K, Xie B, Li S, Rozanski GJ. Regulation of Kv4 channel expression in failing rat heart by the thioredoxin system. American journal of physiology Heart and circulatory physiology. 2008;295:H416–H424. doi: 10.1152/ajpheart.91446.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mittal M, Gu XQ, Pak O, Pamenter ME, Haag D, Fuchs DB, Schermuly RT, Ghofrani HA, Brandes RP, Seeger W, Grimminger F, Haddad GG, Weissmann N. Hypoxia induces Kv channel current inhibition by increased NADPH oxidase-derived reactive oxygen species. Free radical biology & medicine. 2012;52:1033–1042. doi: 10.1016/j.freeradbiomed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 178.Jerng HH, Pfaffinger PJ. S-glutathionylation of an auxiliary subunit confers redox sensitivity to Kv4 channel inactivation. PloS one. 2014;9:e93315. doi: 10.1371/journal.pone.0093315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li H, Chai Q, Gutterman DD, Liu Y. Elevated glucose impairs cAMP-mediated dilation by reducing Kv channel activity in rat small coronary smooth muscle cells. AmJPhysiol Heart CircPhysiol. 2003;285:H1213–H1219. doi: 10.1152/ajpheart.00226.2003. [DOI] [PubMed] [Google Scholar]
- 180.Liu Y, Gutterman DD. The coronary circulation in diabetes: influence of reactive oxygen species on K+ channel-mediated vasodilation. VasculPharmacol. 2002;38:43–49. doi: 10.1016/s1537-1891(02)00125-8. [DOI] [PubMed] [Google Scholar]
- 181.Liu Y, Terata K, Rusch NJ, Gutterman DD. High glucose impairs voltage-gated K(+) channel current in rat small coronary arteries. CircRes. 2001;89:146–152. doi: 10.1161/hh1401.093294. [DOI] [PubMed] [Google Scholar]
- 182.Li H, Gutterman DD, Rusch NJ, Bubolz A, Liu Y. Nitration and functional loss of voltage-gated K+ channels in rat coronary microvessels exposed to high glucose. Diabetes. 2004;53:2436–2442. doi: 10.2337/diabetes.53.9.2436. [DOI] [PubMed] [Google Scholar]
- 183.Heaps CL, Tharp DL, Bowles DK. Hypercholesterolemia abolishes voltage-dependent K+ channel contribution to adenosine-mediated relaxation in porcine coronary arterioles. AmJPhysiol Heart CircPhysiol. 2005;288:H568–H576. doi: 10.1152/ajpheart.00157.2004. [DOI] [PubMed] [Google Scholar]
- 184.Heaps CL, Jeffery EC, Laine GA, Price EM, Bowles DK. Effects of exercise training and hypercholesterolemia on adenosine activation of voltage-dependent K+ channels in coronary arterioles. J Appl Physiol (1985) 2008;105:1761–1771. doi: 10.1152/japplphysiol.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Caouette D, Dongmo C, Berube J, Fournier D, Daleau P. Hydrogen peroxide modulates the Kv1.5 channel expressed in a mammalian cell line. Naunyn-Schmiedeberg’s archives of pharmacology. 2003;368:479–486. doi: 10.1007/s00210-003-0834-0. [DOI] [PubMed] [Google Scholar]
- 186.Platoshyn O, Yu Y, Golovina VA, McDaniel SS, Krick S, Li L, Wang JY, Rubin LJ, Yuan JX. Chronic hypoxia decreases K(V) channel expression and function in pulmonary artery myocytes. Am J Physiol Lung Cell Mol Physiol. 2001;280:L801–L812. doi: 10.1152/ajplung.2001.280.4.L801. [DOI] [PubMed] [Google Scholar]
- 187.Wang J, Weigand L, Wang W, Sylvester JT, Shimoda LA. Chronic hypoxia inhibits Kv channel gene expression in rat distal pulmonary artery. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1049–L1058. doi: 10.1152/ajplung.00379.2004. [DOI] [PubMed] [Google Scholar]
- 188.Fountain SJ, Cheong A, Li J, Dondas NY, Zeng F, Wood IC, Beech DJ. K(v)1.5 potassium channel gene regulation by Sp1 transcription factor and oxidative stress. American journal of physiology Heart and circulatory physiology. 2007;293:H2719–H2725. doi: 10.1152/ajpheart.00637.2007. [DOI] [PubMed] [Google Scholar]
- 189.Park SW, Noh HJ, Sung DJ, Kim JG, Kim JM, Ryu SY, Kang K, Kim B, Bae YM, Cho H. Hydrogen peroxide induces vasorelaxation by enhancing 4-aminopyridine-sensitive Kv currents through S-glutathionylation. Pflugers Arch. 2014 doi: 10.1007/s00424-014-1513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cogolludo A, Frazziano G, Cobeno L, Moreno L, Lodi F, Villamor E, Tamargo J, Perez-Vizcaino F. Role of reactive oxygen species in Kv channel inhibition and vasoconstriction induced by TP receptor activation in rat pulmonary arteries. Annals of the New York Academy of Sciences. 2006;1091:41–51. doi: 10.1196/annals.1378.053. [DOI] [PubMed] [Google Scholar]
- 191.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. AmJPhysiol Heart CircPhysiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 192.Svoboda LK, Reddie KG, Zhang L, Vesely ED, Williams ES, Schumacher SM, O’Connell RP, Shaw R, Day SM, Anumonwo JM, Carroll KS, Martens JR. Redox-sensitive sulfenic acid modification regulates surface expression of the cardiovascular voltage-gated potassium channel Kv1.5. CircRes. 2012;111:842–853. doi: 10.1161/CIRCRESAHA.111.263525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Adda S, Fleischmann BK, Freedman BD, Yu M, Hay DW, Kotlikoff MI. Expression and function of voltage-dependent potassium channel genes in human airway smooth muscle. JBiolChem. 1996;271:13239–13243. doi: 10.1074/jbc.271.22.13239. [DOI] [PubMed] [Google Scholar]
- 194.Brundel BJ, Van Gelder IC, Henning RH, Tieleman RG, Tuinenburg AE, Wietses M, Grandjean JG, Van Gilst WH, Crijns HJ. Ion channel remodeling is related to intraoperative atrial effective refractory periods in patients with paroxysmal and persistent atrial fibrillation. Circulation. 2001;103:684–690. doi: 10.1161/01.cir.103.5.684. [DOI] [PubMed] [Google Scholar]
- 195.Van Wagoner DR, Pond AL, McCarthy PM, Trimmer JS, Nerbonne JM. Outward K+ current densities and Kv1.5 expression are reduced in chronic human atrial fibrillation. CircRes. 1997;80:772–781. doi: 10.1161/01.res.80.6.772. [DOI] [PubMed] [Google Scholar]
- 196.Ohanyan V, Yin L, Bardakjian R, Kolz C, Enrick M, Hakobyan T, Kmetz JG, Bratz I, Luli J, Nagane M, Khan N, Hou H, Kuppusamy P, Graham J, Fu FS, Janota D, Oyewumi MO, Logan SJ, Lindner JR, Chilian WM. Requisite Role of Kv1.5 Channels in Coronary Metabolic Dilation. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.306642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. AmJPhysiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 198.Carl A, Lee HK, Sanders KM. Regulation of ion channels in smooth muscles by calcium. Am J Physiol. 1996;271:C9–C34. doi: 10.1152/ajpcell.1996.271.1.C9. [DOI] [PubMed] [Google Scholar]
- 199.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirc. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 200.Jackson WF, Blair KL. Characterization and function of Ca(2+)-activated K+ channels in arteriolar muscle cells. AmJPhysiol. 1998;274:H27–H34. doi: 10.1152/ajpheart.1998.274.1.H27. [DOI] [PubMed] [Google Scholar]
- 201.Li PL, Jin MW, Campbell WB. Effect of selective inhibition of soluble guanylyl cyclase on the K(Ca) channel activity in coronary artery smooth muscle. Hypertension. 1998;31:303–308. doi: 10.1161/01.hyp.31.1.303. [DOI] [PubMed] [Google Scholar]
- 202.Zhu S, Han G, White RE. PGE2 action in human coronary artery smooth muscle: role of potassium channels and signaling cross-talk. J Vasc Res. 2002;39:477–488. doi: 10.1159/000067201. [DOI] [PubMed] [Google Scholar]
- 203.White RE, Kryman JP, El Mowafy AM, Han G, Carrier GO. cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BK(Ca) channel activity in coronary artery smooth muscle cells. CircRes. 2000;86:897–905. doi: 10.1161/01.res.86.8.897. [DOI] [PubMed] [Google Scholar]
- 204.Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. CircRes. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- 205.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-Derived Reactive Oxygen Species Dilate Cerebral Arteries by Activating Ca2+ Sparks. CircRes. 2005;97:354–362. doi: 10.1161/01.RES.0000177669.29525.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Asano M, Matsuda T, Hayakawa M, Ito KM, Ito K. Increased resting Ca2+ maintains the myogenic tone and activates K+ channels in arteries from young spontaneously hypertensive rats. Eur J Pharmacol. 1993;247:295–304. doi: 10.1016/0922-4106(93)90198-i. [DOI] [PubMed] [Google Scholar]
- 207.Liu Y, Pleyte K, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in aorta of hypertensive rats. Hypertension. 1997;30:1403–1409. doi: 10.1161/01.hyp.30.6.1403. [DOI] [PubMed] [Google Scholar]
- 208.Liu Y, Hudetz AG, Knaus HG, Rusch NJ. Increased expression of Ca2+-sensitive K+ channels in the cerebral microcirculation of genetically hypertensive rats: evidence for their protection against cerebral vasospasm [In Process Citation] CircRes. 1998;82:729–737. doi: 10.1161/01.res.82.6.729. [DOI] [PubMed] [Google Scholar]
- 209.Wiecha J, Schlager B, Voisard R, Hannekum A, Mattfeldt T, Hombach V. Ca(2+)-activated K+ channels in human smooth muscle cells of coronary atherosclerotic plaques and coronary media segments. Basic ResCardiol. 1997;92:233–239. doi: 10.1007/BF00788518. [DOI] [PubMed] [Google Scholar]
- 210.Lu T, He T, Katusic ZS, Lee HC. Molecular mechanisms mediating inhibition of human large conductance Ca2+-activated K+ channels by high glucose. CircRes. 2006;99:607–616. doi: 10.1161/01.RES.0000243147.41792.93. [DOI] [PubMed] [Google Scholar]
- 211.Tang XD, Garcia ML, Heinemann SH, Hoshi T. Reactive oxygen species impair Slo1 BK channel function by altering cysteine-mediated calcium sensing. NatStructMolBiol. 2004;11:171–178. doi: 10.1038/nsmb725. [DOI] [PubMed] [Google Scholar]
- 212.Liu Y, Terata K, Chai Q, Li H, Kleinman LH, Gutterman DD. Peroxynitrite inhibits Ca2+-activated K+ channel activity in smooth muscle of human coronary arterioles. CircRes. 2002;91:1070–1076. doi: 10.1161/01.res.0000046003.14031.98. [DOI] [PubMed] [Google Scholar]
- 213.Miller AW, Dimitropoulou C, Han G, White RE, Busija DW, Carrier GO. Epoxyeicosatrienoic acid-induced relaxation is impaired in insulin resistance. American journal of physiology Heart and circulatory physiology. 2001;281:H1524–H1531. doi: 10.1152/ajpheart.2001.281.4.H1524. [DOI] [PubMed] [Google Scholar]
- 214.Prieto D, Rivera L, Benedito S, Recio P, Villalba N, Hernandez M, Garcia-Sacristan A. Ca2+-activated K+ (KCa) channels are involved in the relaxations elicited by sildenafil in penile resistance arteries. Eur J Pharmacol. 2006;531:232–237. doi: 10.1016/j.ejphar.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 215.Nishijima Y, Zhang DX. H2O2-Induced Dilation in Human Adipose Arterioles: Role of Smooth Muscle K+ Channels. FASEB J. 2015;29:637.4. [Google Scholar]