Regionally Compartmentalized Resident Memory T Cells Mediate Naturally Acquired Protection Against Pneumococcal Pneumonia (original) (raw)
. Author manuscript; available in PMC: 2017 Dec 21.
Published in final edited form as: Mucosal Immunol. 2017 May 17;11(1):220–235. doi: 10.1038/mi.2017.43
Abstract
As children age, they become less susceptible to the diverse microbes causing pneumonia. These microbes are pathobionts that infect the respiratory tract multiple times during childhood, generating immunological memory. To elucidate mechanisms of such naturally-acquired immune protection against pneumonia, we modeled a relevant immunological history in mice by infecting their airways with mismatched serotypes of Streptococcus pneumoniae (pneumococcus). Previous pneumococcal infections provided protection against a heterotypic, highly virulent pneumococcus, as evidenced by reduced bacterial burdens and long-term sterilizing immunity. This protection was diminished by depletion of CD4+ cells prior to the final infection. The resolution of previous pneumococcal infections seeded the lungs with CD4+ resident memory T (TRM) cells, which responded to heterotypic pneumococcus stimulation by producing multiple effector cytokines, particularly IL-17A. Following lobar pneumonias, IL-17-producing CD4+ TRM cells were confined to the previously infected lobe, rather than dispersed throughout the lower respiratory tract. Importantly, pneumonia protection also was confined to that immunologically-experienced lobe. Thus, regionally localized memory cells provide superior local tissue protection to that mediated by systemic or central memory immune defenses. We conclude that respiratory bacterial infections elicit CD4+ TRM cells that fill a local niche to optimize heterotypic protection of the affected tissue, preventing pneumonia.
Keywords: CD4+ T cells, Interleukin 17, Pneumococcus, Pneumonia, Resident Memory Cells
INTRODUCTION
Pneumonia remains a serious public health burden both in the United States and globally. More than 1 million children under the age of 5 die worldwide from pneumonia and associated complications each year 1. In the United States, pneumonia is the most common reason for the hospitalization of children 2 and accounts for nearly half of the infectious disease-related hospitalizations and deaths of older adults 3. Pneumonia disproportionally affects the youngest and older members of the population, with differential underlying immunological explanations 4. The most common cause of community-acquired bacterial pneumonia at both ends of the age spectrum is Streptococcus pneumoniae (pneumococcus). Colonization of the upper airways by pneumococcus is prevalent and recurrent for children and a precursor for pneumococcal disease, which in addition to pneumonia can also include meningitis, sepsis, and otitis media 1,5. Widespread vaccination programs with the pneumococcal conjugate vaccine have significantly reduced the incidence of pneumococcal disease, however this vaccine is by design only capable of protecting against a small subset of pneumococci (so-called “vaccine type”) and some studies report an increase in disease caused by non-vaccine serotypes 5. Challenges with current vaccines highlight the need for a better understanding of protective immune mechanisms in order to develop new vaccines that provide broader protection.
Pneumococcal carriage decreases during the first 2 years of life due in part to the development of naturally acquired adaptive immune memory 6. To provide protection against respiratory pathogens that exhibit substantial diversity within species, such as the seasonal variation in influenza viruses or the >90 different serotypes of pneumococcus currently circulating, naturally-acquired adaptive immune protection must involve heterotypic responses to epitopes widely conserved within a species. Humans have heterotypic memory T cells and serum antibodies that recognize diverse strains of influenza virus7–9 as well as multiple serotypes of pneumococcus7, 10–12. Both epidemiologic and experimental evidence in mice and in humans demonstrate that this naturally-acquired heterotypic immunological memory provides substantial protection against respiratory infection with newly encountered influenza viruses7, 8,13. Very recently, naturally-acquired heterotypic immunity against pneumococcus has been modeled in mice, revealing that CD4+ Th17 cells can help protect the lung against pneumococcal infection14. It remains unclear which types of memory T cells may provide such heterotypic immunity against pneumococcus in the lung, and how they enhance lung defense.
In addition to systemic immune responses, the mucosal surfaces also contain resident memory T cells (TRM) that can be elicited by viral and chronic infections15–18. The first evidence for TRM cells in the lung came from mouse studies which demonstrated that influenza infections result in lung-localized, non-circulating, influenza-specific memory CD4+ T cells that provide superior host defense against subsequent infections compared to the circulating influenza-specific central memory CD4+ T cells19–21. Adult human lungs contain large numbers of CD4+ TRM, cells based on surface staining with CD69, and at least some of these cells respond to influenza, which suggests that they resulted from prior respiratory infection22, 23. Upon stimulation, lung CD4+ TRM cells express a variety of cytokines, perhaps reflecting diverse specificities and functions22, 23. Whether and how the bacterial causes of pneumonia elicit or are influenced by lung CD4+ TRM cells is, to our knowledge, largely unexplored. The types of pathogens recognized by lung CD4+ TRM cells, the responses of lung CD4+ TRM cells to relevant activation stimuli, and the functional capabilities of lung CD4+ TRM cells require further study, with knowledge gaps especially significant for bacterial pneumonia.
RESULTS
Repeated respiratory infections establish heterotypic protection against pneumococcal pneumonia
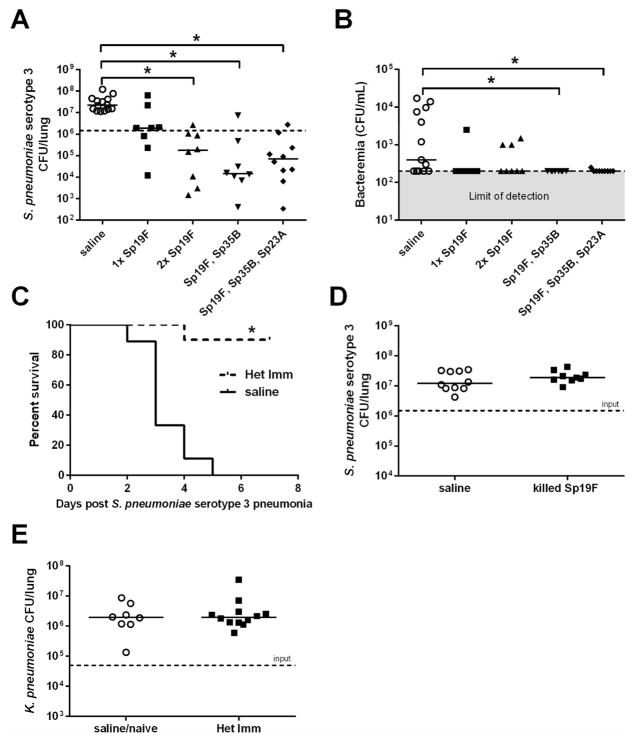
In order to advance understanding of immune mechanisms protecting normal healthy adults from pneumococcal pneumonia, we endeavored to model naturally-acquired heterotypic lung immunity in mice. We caused mild and self-limiting respiratory infections with pneumococcus, allowed 4–8 weeks for any inflammation to subside and then infected the lungs of these mice with Sp3, a serotype to which they had not prevously been exposed. In naïve mice, this Sp3 challenge causes a severe pneumonia which includes inexorable growth of the bacteria in the lungs and dissseminated extrapulmonary infection24, 25. The initial infections were with live pneumococcus via an intranasal (i.n.) instillation to mimic natural infection, using a volume and delivery designed to distribute throughout the upper and lower airways of the mice. When mice were infected i.n. with one dose of Sp19F 4 weeks prior to Sp3 pneumonia challenge, the bacterial burden in the lungs of the mice did not differ significantly from the bacterial burden in the lungs of the control mice treated i.n. with sterile saline (Figure 1A). However, when mice were infected i.n. with two doses of Sp19F one week apart prior to the i.t. Sp3 challenge, there was a multi-log reduction in lung bacterial burden compared to the saline-treated control group (Figure 1A). During early childhood, multiple different pneumococcal serotypes infect the airways and potentially generate immune responses26, 27. We tested whether diversified pneumococcal serotypes were simililarly capable of providing heterotypic immune protection against pneumonia. Serial i.n. infections with Sp19F followed by Sp35B or with Sp19F followed by Sp35B followed by Sp23A were each capable of improving pulmonary defense against a heterotypic pneumococcus compared to control mice that received sterile saline previously instead of pneumococcus (Figure 1A). Bacteremia was also assessed, and mice that received serial pneumococcal exposures had significantly less Sp3 in their blood than control mice previously receiving saline (Figure 1B). These data indictate that prior pneumococcal exposures elicit immune memory that can control and contain a highly virulent pneumococcus after respiratory infection.
Figure 1. Repeated respiratory infections establish heterotypic protection against pneumococcal pneumonia.
C57BL/6 mice were infected with the indicated pneumococcal serotypes (or saline) one, two, or three times intranasally (i.n.) with doses of 1–3×106 CFU each that were seperated by 1 week. After a 4 week rest period mice were challenged with 1×106 Sp3 CFU i.t. for 24h. Lung (A) and blood (B) bacterial burdens were determined. Significance was determined by one-way ANOVA followed by Dunn’s post hoc test. Dashed line indictaes Sp3 CFU infection input. (C) Mice previously exposed to Sp19F, Sp35B, and Sp23A (i.n.) or saline were infected 4 weeks later with 3×105 Sp3 CFU i.t. and followed for 7 days (Kaplan-Meier curve, n=9–10 per group). Significance was determined by Mantel-Cox test. (D) C57BL/6 mice were given 2 doses of heat-killed Sp19F (1×106 CFU) or saline i.n. and challeged 4 weeks later with 1×106 Sp3 CFU i.t. After 24h lung bacterial burden was assessed. (E) Het Imm (i.n.) or saline controls were infected with 5 ×104 Klebsiella pneumoniae CFU i.t. and lung bacterial burden was assessed after 24h. Significance was determined by Mann-Whitney test. Each individual dot represents a single mouse and horizontal lines in scatter plots represent medians. Two independent experiments were performed in every case. *P < 0.05.
In order to determine whether this heterotypic protection was durable and life-saving, we followed cohorts of mice for a longer period of 7 days after Sp3 infection. The saline-treated mice began to decline around day 2 of pneumonia, with no mice living by day 5 (Figure 1C). In contrast, 94% of the Het Imm (i.n.) mice survived to the end of the experiment on day 7 (Figure 1C), at which point they were sacrificed so that lung and blood bacterial burdens could be determined. No Sp3 colonies were recovered from the lungs or blood of nearly all (16/17) mice that had been previously exposed to heterotypic pneumococci. These data demonstrate that heterotypic lung protection generated by mixed exposures to pneumococci can result in long-term sterilizing immunity leading to a full recovery from an otherwise lethal infection.
While our goal was to generate a mouse model of naturally acquired heterotypic anti-pneumococcal pulmonary immunity, there was the potential that respiratory exposures to pneumococcus could bolster non-specific innate immune responses in the lung. In particular, although more transient than the protection observed here (lasting days instead of weeks or months), it has been previously demonstrated that intrapulmonary delivery of non-viable extracts from Haemophilus influenzae bacteria can remodel lung innate immune responses to provide improved host defense that is effective against a wide range of microbes including Gram-positive bacteria, Gram-negative bacteria, viruses, and fungi28. Therefore, we tested whether our pneumococcus-elicited defense against lung infection with heterotypic pneumococci could be generated by dead pneumococci, and whether the protective immunity elicited by respiratory pneumococcal infections extended to bacterial species other than pneumococcus. We did not see the same heterotypic protection against Sp3 pneumonia when mice were treated with equivalent numbers of Sp19F that were heat-killed instead of living (Figure 1D). These data suggest that elements of active infection are essential to the generation of heterotypic immunity in the respiratory tract. Furthermore, prior infections with live pneumococci did not provide lung protection against the unrelated Klebsiella pneumoniae in the lungs (Figure 1E). These data suggest that pneumococcal infections generate protection against pneumonia that is restricted in microbial specificity. Altogether, these results argue against the respiratory exposures to pneumococcus remodeling pulmonary innate immunity alone to protect against a wide range of pathogens, but suggest instead that repeated respiratory infections with pneumococci generate new mechanisms of lung defense that are heterotypic but pneumococcus-specific.
Heterotypic anti-pneumococcal immunity accelerates lung neutrophil recruitment
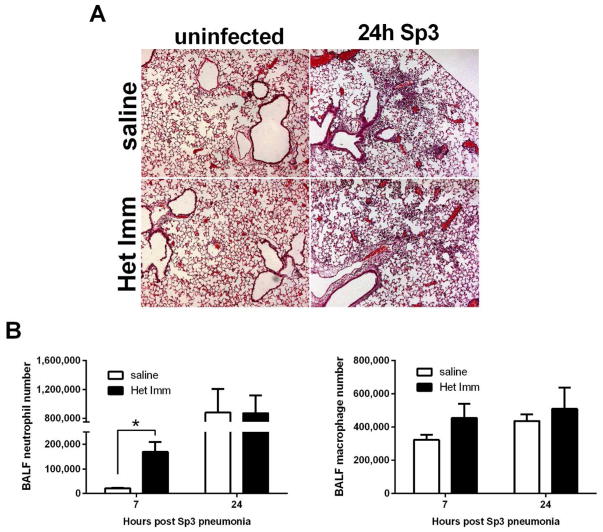
To gain a first glimpse into the mechanisms behind heterotypic lung protection during pneumococcal pneumonia, we began by examining histological views of susceptible and resistant lungs, both with and without Sp3 pneumonia. One mechanism by which viral infections have been demonstrated to remodel adaptive immunity and protect against viral pneumonia is via the generation of inducible bronchus-associated lymphoid tissues (iBALT), characterized by organized lymphoid aggregates in the sub-epithelium and airway interstitium29. However, H&E staining did not reveal iBALT or other types of tertiary lymphoid organs, or any consistent histological differences between non-pneumonic lungs of mice with heterotypic immunity compared to those of saline control mice (Figure 2A). After 24 hours of infection with Sp3, lungs from both groups showed histological evidence of acute pneumonia, including leukocyte recruitment to the air spaces and interstitia (Figure 2A). To quantify the airspace recruitment of leukocytes, bronchoalveolar lavage (BAL) was performed 7 and 24 hours post infection. After 7 hours, there were significantly more neutrophils in the airspaces of the protected lungs compared to susceptible controls (Figure 2B). At 24 hours, a time at which there were multi-log decreases in bacteria in the lungs of mice with heterotypic protection, there were no longer differences in the numbers of airspace neutrophils (Figure 2B). We observed no differences in airspace macrophage numbers between control and protected lungs at either time point. Thus, prior respiratory infections with pneumococcus remodel the lung immune response to accelerate neutrophil recruitment.
Figure 2. Heterotypic anti-pneumococcal immunity accelerates lung neutrophil recruitment.
(A) Representative H&E images of Het Imm and saline control lungs that were collected, fixed with 4% PFA, and paraffin embedded 0 and 24h after an infection with 1×106 Sp3 CFU. Images represent 10X magnification. (B) BALF was collected from Het Imm (i.n.) and saline lungs 7 and 24h following infection with 1×106 Sp3 CFU. Differential cell counts were obtained and data are expressed as total number of neutrophils and macrophages. Bars represent means with standard error of the mean (SEM) displayed. Data were log transformed (Y=Log(Y)) and significance was determined by two-way ANOVA followed by Sidak’s post hoc test (n=8–9 for each group). *P < 0.05 for saline vs Het Imm comparisons. Two independent experiments were performed in every case.
Increased lung-to-neutrophil signaling due to heterotypic immunity
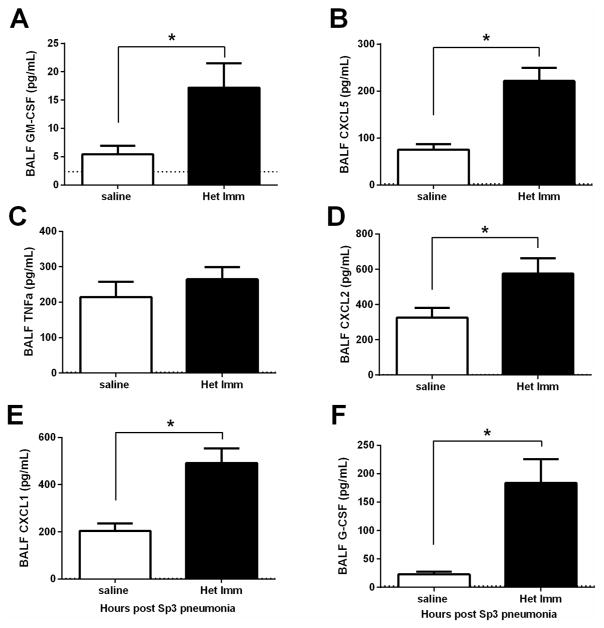
Because neutrophil recruitment was accelerated, we examined the production of neutrophil-directed cytokines in the lungs. During pneumococcal pneumonia, various neutrophil-directed cytokines have been shown to come from distinct cellular sources30, 31. In the airspaces of mice previously exposed to pneumococcus, neutrophil chemokines (CXCL1, CXCL2, and CXCL5) and colony stimulating factors (G-CSF and GM-CSF) were significantly increased in the lungs of mice with heterotypic immunity within 7 hours of the pneumonia challenge (Figure 3). Prior studies indicate the sources of these to be predominantly epithelial cells (CXCL5 and GM-CSF), myeloid cells (CXCL2), or other lung structural cells (CXCL1 and G-CSF)30, 31. These data suggest that during pneumococcal pneumonia in lungs with heterotypic immunity multiple cell types are stimulated to produce higher levels of neutrophil-targeting cytokines.
Figure 3. Increased lung-to-neutrophil signaling due to heterotypic immunity.
(A–G) Het Imm (i.n.) and saline control mice were infected with 1×106 Sp3 CFU and BALF was collected 7 hours later. Cytokine concentrations were measured in the BALF using a multi-plex Luminex assay. Data are expressed as pg/mL and bars represent means with SEM. Dashed lines indicate the limit of detection for the particular analyte. Significance was determined by Student’s t test (n= 14 per group), *P < 0.05. Three independent experiments were performed.
CD4+ Th17 cells in lungs with heterotypic immunity during pneumococcal pneumonia
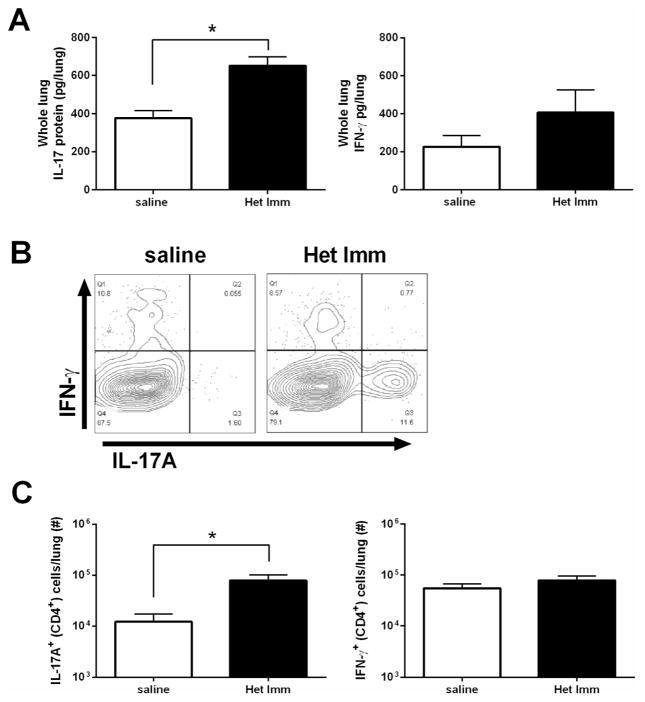
The proinflammatory cytokine IL-17, which can be produced by CD4+ T helper cells (Th17), is a pivotal factor in host defense at mucosal surfaces32, 33. A major effector function of IL-17 is the stimulation of epithelial and stromal cells to produce cytokines that induce the emigration of neutrophils into mucosal sites32, 34. Due to the increase in various neutrophil chemotactic factors in the airspaces of mice with heterotypic protection, we examined the Th17 signal in the lungs of pneumonic naïve and Het Imm mice. We were not able to detect any appreciable IL-17A or IL-23p19, a stablilization factor for Th17 responses35, in the BALF of either treatment group (data not shown). CD4+ T cells are typically found in the lung parenchymal tissue more than in the airspaces20, 22. Therefore, IL-17A protein was measured in whole lung homogenates. Here, IL-17A was detected, and pneumonic lungs from mice with heterotypic immunity contained significantly more IL-17A protein than those from pneumonic naïve mice (Figure 4A). IFN-γ can also enhance neutrophil-mediated defenses against pneumococcus in the lungs36, and like IL-17A can come from memory T cells37. There was no significant difference in IFN-γ content in pneumonic lungs due to heterotypic immunity (Figure 4A). IL-17A can be generated by multiple cellular types, so we tested whether heterotypic immunity caused pneumonic lungs to contain more Th17 cells specifically. Single cell suspensions of pneumonic lungs were stimulated with PMA/Ionomycin plus golgi blockade to allow for intracellular cytokine staining. While the percentage of Th1 (defined as CD4+ IFN-γ+) cells did not change in pneumonic lungs due to previous pneumococcal exposures, the percentage of Th17 (CD4+ IL-17A+) cells was substantially greater in pneumonic lungs with heterotypic immunity (Figure 4B). This impacted the overall abundance of these cytokine-secreting T cells, with significant increases in Th17 cells due to prior serial exposures to pneumococci but no change in the total number of Th1 cells during active heteroypic protection (Figure 4C). Thus, during pneumonia, lungs with heterotypic immunity contain more Th17 cells and IL-17A than do lungs of mice which had never previously experienced pneumococcal respiratory infections.
Figure 4. CD4+ Th17 cells in lungs with heterotypic immunity during pneumococcal pneumonia.
Het Imm (i.n.) and saline control mice were infected with 1×106 Sp3 CFU for 24h. (A) Whole lungs were collected, homogenized and protein was extracted. IFN-γ and IL-17A protein concentrations were measured by ELISA. Data are expressed as pg/lung and bars represent means with SEM. Significance was determined by Student’s t test (n= 10–12 per group). (B) Whole lungs were collected and digested with collagenase to generate single cell suspensions. Cells were stimulated with PMA/Ionomycin for 6h in the presence of a protein transport inhibitor. IL-17A+ and IFN-γ+ CD4+ cells were detected using intracellular cytokine staining (ICS). Representative flow cytometry plots from a Het Imm and saline mouse are displayed. (C) IL-17A+ and IFN-γ+ CD4+ cells were quantified and are displayed as number of cells per lung. Bars represent means with SEM. Significance was determined by Student’s t test (n= 6–7 per group). Two independent experiments were performed in every case. *P < 0.05,
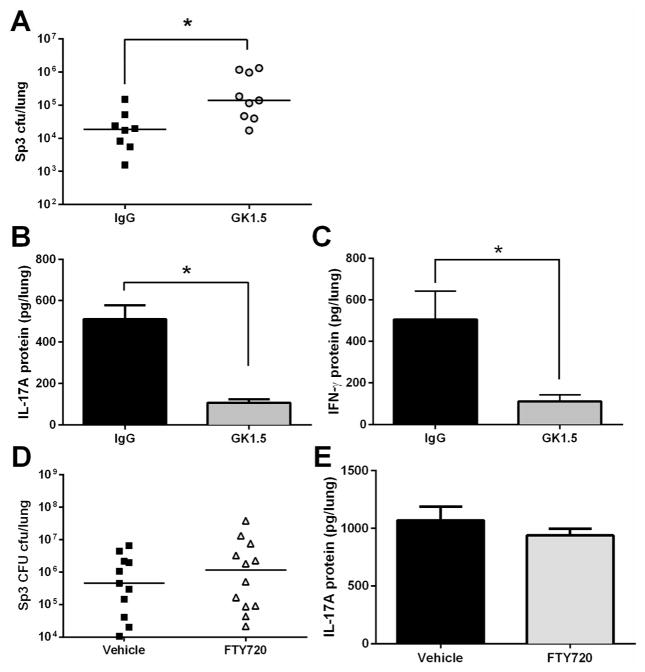
CD4+ Th17 cells can provide defense against pneumococcus14, 38, 39 or other bacteria such as Klebsiella33, making them strong candidates for mediating the protection against pneumonia in the present model of naturally-acquired heterotypic anti-pneumococcal immunity. To test whether CD4+ cells made essential contributions to the lung protection afforded by heterotypic anti-pneumomococcal immunity, we depleted CD4+ cells after full immunity had been established but immediately prior to the final Sp3 challenge. At 72 and 24 hours before the Sp3 pneumonia challenge, mice with heterotypic immunity received administrations of either GK1.5 or IgG intranasally and intraperitoneally in order to ensure complete depletion of CD4+ cells40. Mice that received GK1.5 to deplete CD4+ cells had significantly more Sp3 CFU in their lungs compared to the mice treated with non-targeting IgG (Figure 5A). In addtion, the depletion of CD4+ cells resulted in significantly less IL-17A and IFN-γ protein in the lungs of the GK1.5-treated mice compared to IgG controls (Figure 5B–C). These data indicate that CD4+ cells are critical sources of the increased IL-17A (and also IFN-γ) during pneumonia, and they make essential contributions to the improved lung defense of mice with naturally-acquired heterotypic anti-pneumococcal immunity.
Figure 5. CD4+ cells are required for optimal heterotypic protection against pneumococal pneumonia.
(A) Het Imm mice (i.t.) were administered either a CD4-depleting GK1.5 antibody 72 and 24h before a pneumonia challenge both intranasally (100ug) and intraperitoneally (500ug), or a control IgG antibody at the same concentrations. Mice were infected with 1×106 Sp3 CFU and whole lungs were collected after 24h of pneumonia to determine lung bacterial burden. (B–C) IL-17A and IFN-γ protein levels were measured in the whole lung homogenates from (A). Data were expressed as pg/lung. Significance was determined by Student’s t test. (D) FTY720 or vehicle control was administered to Het Imm (i.t.) mice at a dose of 1mg/kg 2 days prior and the day of a pneumonia challenge with 1×106 Sp3 CFU. Bacterial burden was assessed in whole lungs after 24h. Significance was determined using a Mann-Whitney test. (E) IL-17A protein levels were measured in the whole lung homogenates from (D). Significance was determined by Student’s t test. Individual dots represent a single mouse and horizontal lines represent medians. Dashed line indicates Sp3 CFU infection input. Bars represent means with SEM. Two independent experiments were performed. * P < 0.05.
To test whether this CD4+ T cell-mediated lung defense required lymph node egress characteristic of recirculating memory T cells, we treated mice with the S1P receptor inhibitor FTY720, beginning 4–6 weeks after the initial infections and 2 days prior to and the day of the final pneumonia challenge. FTY720 had no effect on lung bacterial burdens or on lung IL-17A protein levels (Figures 5D–E). Therefore, we conclude that lymph node egress and CD4+ T cell recirculation are not essential for the immune protection in the lungs that is elicited by prior lower respiratory infections.
Recovery from respiratory infection seeds the lungs with pneumococcus-specific Th17 and Th1 cells
The nature and activities of pneumococcus-specific memory CD4+ T cells elicited by respiratory infections are unknown. Because healthy normal human lungs contain resident memory T cells and mice who recover from influenza infection have lungs containing influenza-specific CD4+ TRM cells, we hypothesized that recovery from respiratory infections with pneumococcus may leave behind a population of pneumococcus-specific CD4+ T cells in the lungs, poised to respond to subsequent infections with the production of protective cytokines20, 22, 23, 41. Therefore, we investigated pneumococcus-specific CD4+ T cells from healthy lungs, in which no pneumonia or other infectious process was underway.
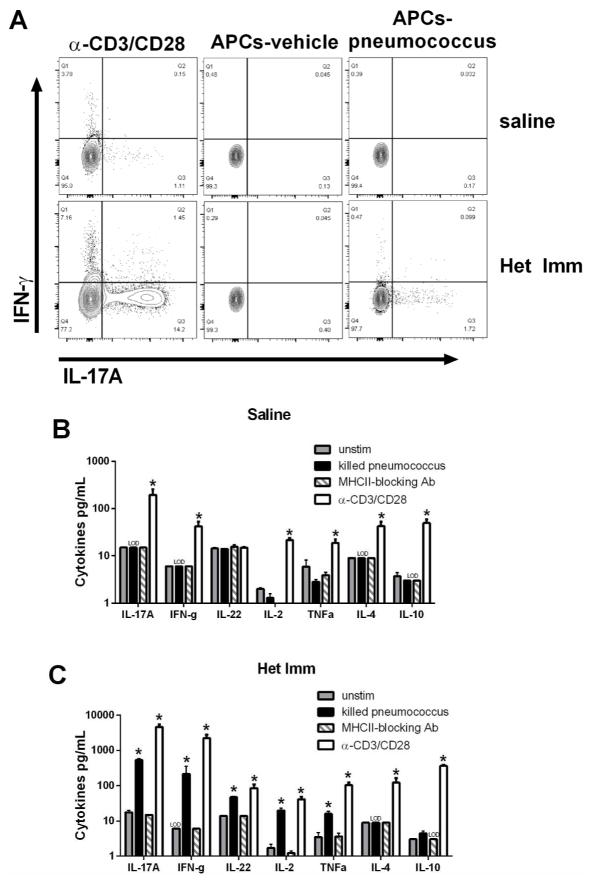
We collected CD4+ cells from the healthy and histologically normal (Figure 2A) lungs of mice with or without heterotypic immunity, and stimulated these cells with pneumococcus-pulsed APCs to assess reactivity. To monitor cell-specific expression of cytokines that defend the lungs, we used intracellular cytokine staining to measure IL-17A and IFN-γ in CD4+ T cells. Non-specific stimulation of T cells using anti-CD3/anti-CD28 beads demonstrated that both types of lungs contained CD4+ T cells that were capable of elaborating these cytokines (Figure 6A, left), consistent with prior analyses of mouse and human lungs19, 23. Neither cytokine was produced by CD4+ T cells from either type of lung when they were cultured with vehicle-pulsed APCs (Figure 6A, middle). Importantly, both IL-17A and IFN-γ were expressed by CD4+ T cells from the lungs of previously-infected mice that were cultured with pneumococcus-pulsed APCs (Figure 6A, right). These data demonstrate for the first time that pneumococcus-specific CD4+ T cells are in the lungs of mice with heterotypic immunity, even without an ongoing infection. Of interest, these cells are not polyfunctional with regard to the two cytokines analyzed, but instead appear to separate into distinct sets with Th1-like or Th17-like properties.
Figure 6. Recovery from respiratory infection seeds the lungs with pneumococcus-specific Th17 and Th1 cells.
The left lobes of Het Imm (i.t.) and saline control mice were digested with collagenase and CD4+ cells were stimulated with killed Sp3-pulsed splenocytes. (A) After 12h of stimulation in the presence of a protein transport inhibitor cells were harvested and CD3+ cells were analyzed for the production of IL-17A and IFN-γ by intracellular cytokine staining. Flow cytometry plots show the percentage of IL-17A and IFN-γ production from CD3+ cells in 3 groups: pan T cell activator α-CD3/CD28 (left), vehicle-pulsed antigen presenting cells (APCs, middle), and killed pneumococcus-pulsed APCs (APCs-pneumococcus, right). Two independent experiments were performed. (B–C) CD4+ cells from Het Imm and saline lungs were stimulated with killed Sp3-pulsed splenocytes for 72h. Supernatants from saline (B) and Het Imm (C) stimulations were collected and cytokine concentrations were measured using a multi-plex luminex assay. In addition to killed pneumococcus three controls are displayed: vehicle-pulsed splenocytes (untim), pan T cell activator α-CD3/CD28, and α-MHC-II blocking antibody added to killed pneumococcus-stimulated samples. IL-22 protein was measured independently using an ELISA. Cytokine concentrations were measured in 3 separate stimulation experiments and are displayed as pg/mL. Bars represent means with SEM. The limit of detection for each assay is indicated (LOD). Data were log transformed (Y=Log(Y)) and significance was assessed by one-way ANOVA followed by Sidak’s post hoc test, *P < 0.05 vs APCs-vehicle (unstim) for each cytokine. Three independent experiments were performed.
We next sought to determine whether other cytokines that are hallmarks of T cell activation may also be produced by pneumococcus-specific CD4+ T cells, in addition to IL-17A and IFN-γ. To answer this question, cultures of lung CD4+ T cells plus APCs that were pulsed with vehicle or pneumococcus were incubated for 72 hours, after which supernatants were analyzed for concentrations of T helper cell effector cytokines. Blocking antibody targeting MHC-II was used to test whether antigen presentation to CD4+ T cells was specifically essential to the cytokine responses. Figures 6B–C show the results from CD4+ T cells cultured with vehicle-pulsed APCs (unstim), pneumococcus-pulsed APCs (killed pneumococcus), pneumococcus-pulsed APCs plus MHC-II blockade, or anti-CD3/anti-CD28 as a positive control. CD4+ cells from mice without heterotypic immunity were functionally capable of making cytokines as evidenced by anti-CD3/anti-CD28 stimulation, but they produced none of these cytokines in response to pneumococcus-pulsed APCs (Figure 6B). In sharp contrast, CD4+ cells from the lungs of mice with heterotypic immunity produced multiple cytokines in response to pneumococcus-pulsed APCs, including IL-17A, IFN-γ, IL-22, IL-2, and TNF-α (Figure 6C). The induction of these cytokines was completely ablated by the MHC-II blockade (Figure 6C), confirming that this cytokine expression resulted from antigen-specific MHC-II-dependent activation of CD4+ T cells. However, MHC-II-dependent activation of pneumococcus-specific CD4+ cells from lungs with heterotypic immunity did not result in expression of either IL-4 or IL-10 (Figure 6C), demonstrating that T cells residing in the lungs after respiratory infections with pneumococcus have distinct T helper cell phenotypes. Overall, these results demonstrate that respiratory infections seed the lungs with pneumococcus-specific memory CD4+ T cells that include Th17 and Th1 phenotypes.
Lung CD4+ TRM cells and heterotypic pulmonary protection imprint locally
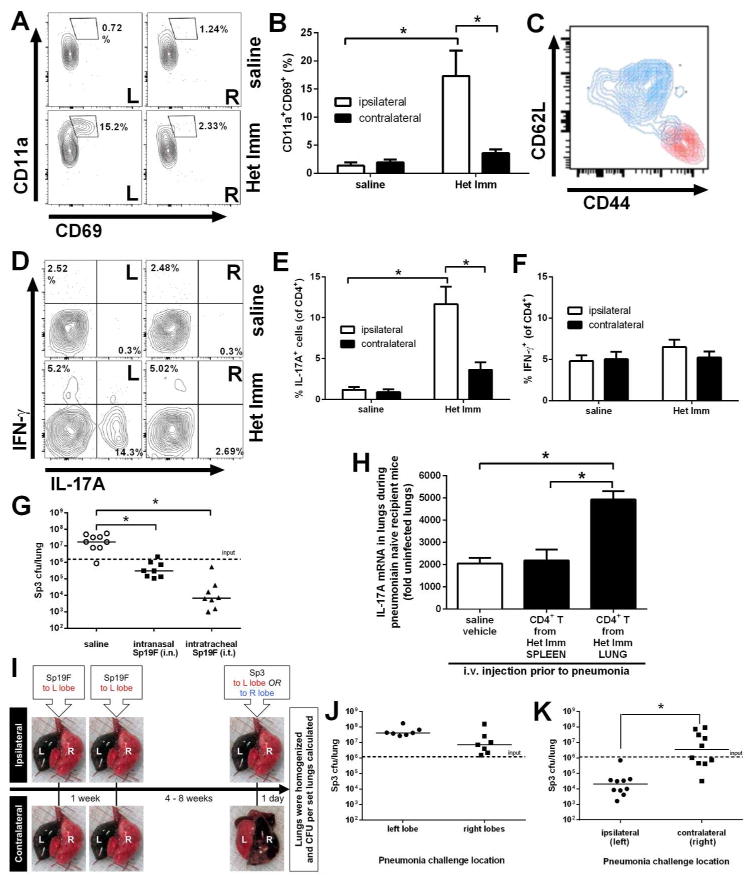
In addition to being non-circulating CD4+ T cells that reside in tissues, resident memory CD4+ cells are defined by high cell surface expression of CD11a and CD69 in the unactivated state19, 20. In TRMmice, CD4+ TRM cells also have high expression of the memory marker CD44 and low expression of the lymphoid homing receptor CD62L20. While TRM cells disperse throughout the skin following a localized viral infection42, nothing is known about regional localization of lung TRM cells after the resolution of a lung infection. This is of particular interest for pneumococcal respiratory infections because they typically result in lobar pneumonia. We modeled this in mice by generating pneumococcal infections in a single lung lobe, and then analyzing CD4+ TRM cells and integrated antibacterial defense in the same (ipsilateral) lung lobe compared to the previously uninfected contralateral lobes from the other side of the thoracic cavity. The ipsilateral lobe showed clear evidence of lung CD4+ TRM cells with a distinct population of CD3+CD4+CD11ahigh CD69+ cells appearing in mice with heterotypic immunity but not in those without (Figure 7A). There were significantly more lung CD4+ TRM cells in the previously infected mice compared to previously uninfected mice (Figure 7B), demonstrating that pulmonary infections with pneumococcus generate lung CD4+ TRM cells. These CD3+CD4+CD11ahighCD69+ cells from the lung were also CD44high and CD62Llow whereas CD3+CD4+CD11alowCD69− cells displayed lower CD44 expression and higher CD62L expression (Figure 7C), further confirming the lung CD4+ TRM cell phenotype. In striking contrast to the ipsilateral lobe, the contralateral lung lobes did not show accumulation of CD3+CD4+CD11ahighCD69+ cells (Figure 7A,B). This leads to the unexpected conclusion that lung CD4+ TRM cells localize to and remain in the previously infected tissue site rather than distributing widely throughout the respiratory mucosa.
Figure 7. Lung CD4+ TRM cells and heterotypic pulmonary protection imprint locally.
The left (L) and right (R) lobes of uninfected Het Imm (i.t.) and saline control mice were digested separately with collagenase. (A) Representative flow cytometry plots show lung TRM cells that were identified as CD3+/CD4+/CD11ahi/CD69+/CD62Llo/CD44hi. (B) TRM cells were quantified in the left (ipsilateral) and right (contralateral) lobes of Het Imm and saline mice. Data are displayed as % CD11ahiCD69+ of CD4+ cells. Bars represent means with SEM. Significance was determined by two-way ANOVA followed by Tukey’s post hoc test (n= 4–5 per group, experiment repeated three times). (C) Representative flow cytometry plot of CD44 and CD62L expression on CD11ahiCD69+ lung TRM cells (red) and lung CD4+CD11aloCD69- cells (blue). (D) Single cells from the left (ipsilateral) and right (contralateral) lobes of Het Imm (i.t.) and saline mice were stimulated with PMA/Ionomycin for 6h in the presence of a protein transport inhibitor. ICS and flow cytometry was performed to monitor cytokine production. Representative flow plots show IL-17A and IFN-γ-producing CD4+ cells from Het Imm and saline lungs. (E–F) IL-17A and IFN-γ-producing CD4+ cells were quantified in the left (ipsilateral) and right (contralateral) lobes of Het Imm and saline mice. Bars represent means with SEM. Significance was determined by two-way ANOVA followed by Tukey’s post hoc test (n= 5–6 per group). Three independent experiments were performed. (G) Mice on a mixed background were infected twice with saline, intranasal Sp19F (Het Imm i.n.), or intratracheal Sp19F (Het Imm i.t.). After 4 weeks mice from each group were challenged with 1 × 106 Sp3 CFU. Lungs were harvested after 24h and bacterial burden was determined. Individual dots represent a single mouse and horizontal lines represent medians. Dashed line indicates Sp3 CFU infection input. Significance was determined by one-way ANOVA followed by Dunn’s post hoc test. (H) CD4+ lymphocytes from the lungs and spleens of uninfected Het Imm were isolated using FACS. Three minutes prior to euthanization intravital cells were fluorescently labeled with anti-CD45-APC antibody via i.v. injection. The cells for transfer were identified as live, i.v. label-negative CD45+CD4+ cells. Naïve recipient mice were administered saline, lung-derived or spleen-derived CD4+ cells (1–4×106 per mouse) from Het Imm mice transferred via i.v. tail vein injection. Three days after the transfer, recipient mice were challenged with 1×106 Sp3 CFU. After 24h, left lung lobes were harvested, mRNA was extracted, and qRT-PCR for IL-17A was performed. Data were displayed as fold induction for each group normalized to uninfected lungs. Significance was determined by one-way ANOVA followed by Tukey’s post hoc test (n=3–5 per group). Bars represent means with SEM. (I) Ipsilateral vs contralateral heterotypic protection model. Het Imm (i.t.) mice received two Sp19F infections in their left lobes. After 4 weeks, half of the mice were challenged with 1×106 Sp3 CFU in their ipsilateral (left) lobe, and the other half challenged in their contralateral (right) lobes. Images represent the instillation (i.t.) of diluted colloidal carbon into C57BL/6 mouse lungs either in the left (L) or right (R) lobes. Lungs were immediately collected and the deposition of the dye was visualized. (J) Naïve C57BL/6 mice were infected with 1×106 Sp3 CFU selectively in either the left or right lobes. Lung bacterial burden was assessed after 24h. (K) The mice described in (I) were challenged with 1×106 Sp3 CFU in the specified location. After 24h whole lungs were collected and bacterial burden was assessed. Individual dots represent a single mouse and horizontal lines represent medians. Dashed line indicates Sp3 CFU infection input. Significance was determined using a Mann-Whitney test. Two independent experiments were performed. * P < 0.05.
To determine whether IL-17A-producing CD4+ T cells were also differentially localized to the site of previous pneumococcal infections, cells from the left and right lung lobes of mice with or without heterotypic anti-pneumococcal immunity were analyzed by intracellular cytokine staining. A distinct popualion of IL-17A-producing cells was present only in the ipsilateral left lobes from mice with heterotypic immunity, but not in the same lobe from control mice nor in the contraleral lobes from those mice with heterotypic immunity (Figure 7D,E). Cells that expressed IFN-γ were not compartmentalized the same way (Figures 7D,F). While previous literature has demonstrated a distribution of memory cells throughout a surface such as the skin following infection42, in our model the IL-17-skewed lung TRM cells remain at the site of previous pneumococcal infections.
If localized lung CD4+ TRM cells are responsible for anti-pneumococcal defense of the tissues, then pulmonary defense against severe pneumonia should also localize to the previously infected lung tissues. Studies described above revealed that both i.n. and i.t. infections with pneumococcus were capable of eliciting heterotypic anti-pneumococcal pulmonary defense. The former more diffusely distributes the bolus of bacteria throughout the respiratory tract including the upper airways and all 5 lung lobes, whereas the latter concentrates all of the infection to a single lung lobe. Both i.n. and i.t. pneumococcal exposures resulted in immune defenses that led to a significant reduction in lung Sp3 burden compared to saline control mice, with the more concentrated infections trending towards (but not reaching statistical significance) even better protection (Figure 7G). These data demonstrate that a natural route of infection with pneumococci accessing the lungs via the nasopharyngeal cavity can elicit lower repiratory tract protection. We used an adoptive transfer protocol to test whether CD4+ T cells from the lungs had distinct abilities to transfer immune activities. Extravascular CD4+ T cells were sorted from the lungs and spleens of mice with heterotypic immunity, and were injected i.v. into naïve recipient mice. A heterotypic strain of pneumococcus was subsequently delivered to the lungs of these naïve recipients, and after 24 hours IL-17 mRNA was measured in the infected lung. The transfer of spleen CD4+ T cells did not change lung IL-17 expression compared to negative vehicle control, but the transfer of lung CD4+ T cells was sufficient to significantly increase IL-17A expression in the pneumonic lung (Figure 7H). These results provide evidence that lung-resident CD4+ T cells are superior to spleen-derived CD4+ T cells in providing heterotypic pulmonary immunity during pneumonia.
Given our findings that lobar pneumonia led to a lobe-specific accumulation of lung CD4+ TRM cells, we hypothesized that heterotypic protection against pneumococcus might also be restricted within the respiratory tract. In order to test whether the compartmentalization of TRM cells within the lung could impact heterotypic lung protection, we designed a unique animal model in which the initial and final infections could be matched to the same lobe or delivered instead contralaterally (Figure 7I). To determine whether anatomical or other differences between the left and right lobes of the mouse lung might impact lung defense in the absence of prior infections, naïve mice were examined. Anatomical differences between the left and right lung lobes did not affect bacterial growth as Sp3 lung burdens were equivalently high in naïve mice whether the infection was in the left or right lobes (Figure 7J). Groups of mice were infected twice with Sp19F i.t. in their left lobe selectively, separated by a week, and then after 4–8 weeks one group was challenged with Sp3 i.t. in the ipsilateral (left) and the other group in the contralateral (right) lobes (Figure 7I). After 24h of lobar pneumonia, lungs were harvested and bacterial burdens were measured. In stark contrast to the naïve mice (Figure 7J), in mice which had recovered from prior heterotypic infections of the left lobe, infections of the left (ipsilateral) lobe led to significant decreases in bacterial burden compared to infections of the right (contralateral) lobes (Figure 7K). The previously infected lobe but not the contraleral lobes were capable of initiating a rapid and robust heterotypic recall response that protected against further infection. We conclude that a localized pneumococcal infection in the lung results in the regional deposition of CD4+ TRM cells that protect the immediate but not distant respiratory mucosa against a subsequent heterotypic pneumococcal pneumonia.
DISCUSSION
Repeated respiratory infections with pneumococcus establish heterotypic immune protection against pneumococcal pneumonia, mediated by regionally compartmentalized lung CD4+ TRM cells. We propose that this mimics the naturally acquired immune protection that is afforded most healthy young adult humans, but which wanes over time due to aging and co-morbidities such as smoking, alcohol abuse, and chronic disease43–45. Incorporating the model of naturally acquired heterotypic immune protection against pneumonia with other mouse models of aging and co-morbidity will facilitate the investigation of mechanisms underlying pneumonia susceptibility in older adults, which is a major contributor to unhealthy aging46. The present studies suggest that the transition from a susceptible young child to a more protected adolescent or young adult involves the regional establishment of lung CD4+ TRM cells recognizing respiratory pathobionts.
Prior to pneumococcal infection, adult laboratory mice do not have CD4+ TRM cells in their lungs, as seen both here and in previous studies19. This contrasts markedly from adult human lungs, in which CD4+ TRM cells are consistently abundant22, 23. Repeated pneumococcal infections of the respiratory tract seed the lungs with antibacterial TRM cells, and similar consequences result from respiratory virus infections20, 47. It appears that lower respiratory tract infection, rather than the microbiome (which is present in laboratory mice), is essential to generating lung CD4+ TRM cells.
The cleanliness of the environments in which laboratory mice are raised and studied is an important limitation to mouse models47, 48. Immune responses in standard laboratory mice may most closely model the immune responses of infants and young children, which are important to understand since early childhood is an especially vulnerable time47, 48. However, to use mice for modeling and studying immune responses to pathogens in adult humans, it may be necessary to first create a relevant infection history. In this communication, we utilize models that are relevant to studying the naturally-acquired heterotypic immune mechanisms that protect most young healthy adults against pneumococcal infection. Similar adaptations for other common respiratory pathobionts should also be established, so that the research community will be better empowered to study the immunoprotective mechanisms most important to the majority of the population.
Consistent with our own work, a recent study demonstrates that recovery from prior pneumococcal infections generates memory CD4+ cells that are essential for cross-serotype host protection14. Here, we demonstrate for the first time that CD4+ TRM cells have a predominant role in mediating host defense during pneumococcal pneumonia. Studies of both human and mouse lungs have observed influenza-specific TRM cells20, 22, but the data presented here are to our knowledge the first evidence of lung TRM cells specific to any of the bacterial causes of pneumonia. Like the pneumococcus, most of the microbes that most commonly cause pneumonia are ubiquitous pathobionts49. We postulate that lung TRM cells may be critical to preventing pneumonias from diverse etiologies.
The localization of TRM cells at the anatomical site of possible reinfection is a fundamental aspect of tissue-resident memory and rapid recall responses, but the distribution of these cells within a complex organ like the lung is largely unknown at present. One study of localized vaccinia virus skin infection in mice found that a population of CD8+ non-circulating TRM cells resided not only at the initial site of infection but also distributed throughout the entire skin surface42. Another study found that memory CD8+ cells preferentially persisted in the epidermis at the site of prior infection in a mouse model of cutaneous herpes simplex virus-1 (HSV) infection50, migrating only slowly in a random fashion and so remaining localized within a few millimeters of the site of initial infection50. The differences in TRM distribution between these two studies could be due to the different infection courses by two different viruses. To our knowledge, we are the first to show the regional localization of lung TRM cells, and we have leveraged this to demonstrate a profound regional impact on memory recall responses and the outcome of reinfection. Het Imm mice that were challenged contralateral to the initial infections were unable to control the infection and displayed very little heterotypic protection, while mice challenged in the ipsilateral lobe possessed full heterotypic protection. Systemic mechanisms of protection, such as central memory CD4+ T cells and circulating anti-pneumococcal antibodies, should be equally protective for ipsilateral and contralateral lung lobes. While the actions of anti-pneumococcal TRM cells likely synergize with systemic arms of immunity, the co-localization of heterotypic lung protection with lung TRM suggests that tissue-resident immune memory is an essential mechanism of protection that results from the resolution of respiratory infection.
The effector functions of TRM cells generated following resolution of pneumococcal infections appear complex and to involve multiple parallel protective pathways. When CD4+ TRM cells from the lungs of mice previously exposed to pneumococcus were stimulated in an antigen-specific manner they produced not only IL-17A but also IFN-γ, IL-2, TNF-α, and IL-22. IL-17A has been shown in many circumstances to be protective during both colonization and infection with pneumococcus, through the induction of neutrophil recruitment14, 38, 39. In addition to T helper cells, there are also innate sources of IL-17 like gamma-delta (γδ) T cells and group 3 innate lymphoid cells (ILC3). In a Klebsiella vaccination study,γδ T cells were the main source of lung IL-17 during a primary infection, but respiratory tract vaccination led to CD4+ T cells emerging as the dominant source during Klebsiella pneumonia33. In the context of pneumococcal pneumonia, lung ILC3s have been shown to produce IL-17 during a primary infection51. While we cannot at this time rule out the possibility that there are additional cellular sources of IL-17, our CD4+ cell depletion data suggest that CD4+ T cells are the major source of IL-17 in the lungs of mice with heterotypic immunity.
Many of the other cytokines we identify here as products of pneumococcus-specific lung CD4+ TRM cells may further contribute to protection. IFN-γ stimulation of macrophages induces antimicrobial programs and enhances antigen processing and presentation37. In addition, IFN-γ facilitates bacterial clearance during pneumococcal pneumonia through the augmentation of neutrophil extracellular trap formation36. IL-22 stimulates the production of antimicrobial peptides by the airway epithelium during Gram-negative bacterial pneumonia52. TNF-α signaling during pneumococcal pneumonia amplifies NF-κB activation throughout the infected lung, improving neutrophil recruitment and bacterial clearance24. IL-2 is an important cytokine for T cell proliferation, homeostasis, and differentiation53, and it may be critical in this setting for helping establish and maintain lung resident memory cells, as has been observed in the setting of allergic airways disease54. A shared downstream mechanism for many from this group of inflammatory modulators involves the acceleration of neutrophil recruitment and amplification of phagocyte function. This functional redundancy during heterotypic lung protection may prevent bacteria from easily developing resistance against these immune mechanisms.
The goal of the work presented here was to increase our understanding of the naturally acquired immune mechanisms that mediate rapid recall responses in the lung. This led to improved mouse models for studying immunity in the lungs, and the demonstration that the regional accumulation of lung CD4+ TRM cells plays a predominant role in preventing the most common cause of bacterial pneumonia. Many exciting and important questions remain. The biology of lung CD4+ TRM cells is only beginning to be elucidated, and the signals that recruit and retain these cells in the tissue remain to be determined. Tracking the number and function of lung TRM cells in settings of susceptibility such as aging or co-morbidities will provide valuable mechanistic insights into pneumonia risk. Future vaccine strategies that generate lung TRM cells against potential respiratory pathogens like pneumococcus may be novel approaches for protecting individuals developing susceptibility due to advancing age or co-morbidities. Activating these lung TRM cells pharmacologically to produce downstream immune effectors that provide antimicrobial defense could be an additional innovative approach to pneumonia therapy. Taking informed steps in these exciting directions will require improved knowledge of antimicrobial lung CD4+ TRM cells.
METHODS
Mice
All animal protocols were approved by the Boston University Institutional Animal Care and Use Committee. Experiments were initiated when mice were 6–15 weeks of age. Female C57BL/6J mice were used unless otherwise specified, and were purchased from Jackson Laboratories (Bar Harbor, Maine). Mice were maintained in specific pathogen-free conditions at Boston University Medical Center (BUMC).
Heterotypic Immunity
Bacteria were grown on blood agar plates (Trypticase Soy Agar (TSA II) with 5% Sheep Blood, BD Biosciences) for 14 hours at 37°C with 5% CO2 and suspended in sterile saline prior to infecting mice. For heterotypic immunity, mice were serially infected with 1–3 (as specified) doses of 1–3×106 colony forming units (CFU) Streptococcus pneumoniae serotype 19F (EF3030, Sp19F), serotype 35B (Sp35B), and/or serotype 23A (Sp23A), with one week intervals between such serial infections. Such infections were self-limiting. Sp23A and Sp35B were clinical isolates provided by Dr. Stephen Pelton (Boston University). Intranasal (i.n.) instillations were performed by anesthetizing mice with a ketamine (50mg/kg) and xylazine (5mg/kg) mixture via intraperitoneal (i.p.) injection and applying a 10μL bacteria suspension to each naris until the inoculation was inhaled completely. These mice are referred to as Het Imm (i.n.); control mice received i.n. instillations of sterile saline. In some experiments, as specified, mice were infected intratracheally (i.t.) as previously described55 in the left lobes with two doses of Sp19F one week apart; control mice received i.t. sterile saline instillations. Infections were with 1–3 ×106 CFU per mouse for all serotypes and routes of delivery. For heat-killed Sp19F, bacteria were suspended at a dose of 1–3 ×106 CFU per mouse and incubated in a 55°C water bath for 45 minutes prior to i.n. instillation; plating confirmed no living Sp19F. Mice were rested 4 to 8 weeks after the final infection or saline instillation before being analyzed further or enrolled in pneumonia experiments.
Pneumonia
Streptococcus pneumoniae serotype 3 (Sp3, ATCC 6303) was instilled i.t. at a dose of 1×106 CFU unless otherwise stated for pneumonia challenges. Klebsiella pneumoniae (ATCC 43816) was instilled i.t. at a dose of 5×104 CFU per mouse. To assess bacterial burden, mice were euthanized at specified time points by isoflurane overdose, and CFU were enumerated in lungs that were homogenized (Bullet blender, Next Advance) in water containing a protease inhibitor cocktail (Roche, Risch-Rotkreuz, Switzerland) as well as in blood samples collected from the inferior vena cava. When indicated, mice received FTY720 i.p. at a dose of 1 mg/kg 2-days prior to and on the day of final pneumonia challenge56. The efficacy of the FTY720 regimen was confirmed by observation of decreased peripheral blood CD4+ T cells.
Lung Histology
After heart ligation, the lungs were fixed by inflation with 4% paraformaldehyde at 23 cm H20 pressure. Left lobes were paraffin embedded, cut into 5 μM sections, and stained with hematoxylin/eosin57.
Bronchoalveolar Lavage
Bronchoalveolar lavage fluid (BALF) was collected as previously described55. Briefly, lungs were removed and secured to a blunted 20-gauge needle via the trachea and inflated with 1mL ice cold phosphate buffered saline (PBS), after which the liquid was withdrawn and collected. The process was repeated until the lungs had been lavaged with a total of 10mL PBS. The first milliliter was collected in a separate syringe and saved for protein analysis. Cells were counted using a hemocytometer and cell differentials were obtained using a cytocentrifuge followed by staining with Diff-Quik solutions (Dade-Behring).
Cytokine concentrations
Cytokine concentrations in BALF were assessed using a Bio-plex magnetic bead assay (Luminex, R&D) and analyzed using a Bio-Plex 200 workstation (Bio-Rad). The cytokine panel included granulocyte-macrophage colony-stimulating factor (GM-CSF), granulocyte colony-stimulating factor (G-CSF), CXCL1, CXCL2, CXCL5, CCL20, IL-23p19, IL-17A, and TNF-α. Whole lung IL-17A and IFN-γ protein concentrations were measured by enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minneapolis, MN) following protein extraction from lung homogenates58. Cytokine concentrations in ex vivo CD4+ T cell stimulation supernatants were measured by Luminex (for IL-17A, IFN-γ, IL-2, TNF-α, IL-4, and IL-10; R&D Systems) or ELISA (for IL-22; R&D Systems).
Lung cell suspensions
To generate single-cell suspensions, mouse lungs were digested in Type 2 collagenase (Worthington Biochemicals, Lakewood, NJ). Lung lobes were isolated and stored in RPMI-1640 media (Life Technologies, Carlsbad, CA) on ice until minced in digestion solution (Type 2 collagenase 1mg/mL, DNase I 150μg/mL, CaCl2 2.5mM in PBS), incubated at 37°C while shaking for 1 hour, and passed through a 70μm cell strainer (Fisher, Grand Island, NY). Erythrocytes were removed using red blood cell lysis buffer (Sigma, St. Louis, MO) and single-cell suspensions were counted using a hemocytometer.
CD4+ Depletion
For CD4+ depletion studies Het Imm (i.t.) mice were administered an anti-CD4+ monoclonal antibody (clone GK1.5) or a rat IgG2b isotype control antibody (BioXcell, West Lebanon, NH). Antibodies were diluted in sterile saline and delivered at doses of 500 μg i.p. and 100μg i.n. (under anesthesia) both 72 and 24 hours prior to the i.t. Sp3 pneumonia challenge.
Ex vivo PMA/Ionomycin stimulation of lung cells
Cells from lungs digested using the collagenase single-cell suspension protocol were resuspended in RPMI-1640 media containing 10% fetal bovine serum (FBS), penicillin (100U/mL)/streptomycin (100 μg/mL), 1mM sodium pyruvate, 0.2μM 2-mercaptoethanol, 2mM L-glutamine, 0.1mM non-essential amino acids, and 10mM HEPES buffer and cultured at 3×106 cells/well in 6-well cell culture-treated plates (Corning, Corning, NY), with cells from each mouse incubated separately in their own well. PMA (LC Laboratories, Woburn, MA) at a final concentration of 100 ng/mL and ionomycin (Sigma, St. Louis, MO) at a final concentration of 1500 ng/mL were used to stimulate the cells for 1 hour at 37°C with 5% CO2 before GolgiStop (BD Biosciences, San Jose, CA) was added; cells were cultured an additional 4 hours. Intracellular cytokine staining (ICS) was performed using the BD Cytofix/Cytoperm Fixation/Permeabilization kit (BD Biosciences, San Jose, CA) and antibodies to label the following cell surface markers and cytokines were used: CD45 (clone 30-F11, Biolegend, San Diego, CA), CD3ε (clone 145-2C11, Biolegend), CD4 (clone GK1.5, eBioscience, San Diego, CA), IL-17A (clone eBio17B7, eBioscience), and IFN-γ (clone XMG1.2, eBioscience). Stained cells were analyzed using a LSRII (BD Biosciences, San Jose, CA) and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
Pneumococcus-specific T cell responses
CD4+ cells were positively selected using the EasySep positive selection kit (StemCell Technologies, Vancouver BC, Canada) from left lobes of saline and Het Imm (i.t.) mice that were digested according to the collagenase single-cell suspension protocol; cells from 3 saline mice or Het Imm (i.t.) mice were pooled for each experiment. Antigen-presenting cells (APCs) were collected from spleens of naïve C57BL/6 mice that were pushed through a 70μm cell strainer, washed, and rid of red blood cells. APCs were pulsed with killed-Sp3 (kSp3) by incubating at a ratio of 3:1 (bacteria:cells) at 37°C with 5% CO2 for 1 hour with gentle agitation every 20 minutes. The kSp3 preparation was generated using beta-propiolactone (BPL) as a bactericidal agent, as previously described59. Some splenocytes were vehicle-pulsed (no killed Sp3) as unstimulated APC controls. Mitomycin C (Fisher Scientific, Waltham, MA) was added to prevent splenocyte proliferation. After washing three times, pulsed splenocytes were mixed with CD4+ cells at a 10:1 ratio of splenocytes:CD4+ cells. For positive controls, CD4+ cells were stimulated with mouse T-activator CD3/CD28 Dynabeads® (Life Technologies, Carlsbad, CA) at a 3:1 bead:cell ratio. Purified anti-mouse MHC-II clone M5/114.15.2 and isotype control clone RTK4530 (Biolegend, San Diego, CA) were added to indicated wells at a concentration of 10 μg/mL. For cytokine concentrations, cells were incubated for 72 hours, after which supernatants were collected and stored at −80°C until multi-plex protein analyses. For ICS assays, cells were cultured 12 hours in the presence of GolgiStop, and splenocytes had a mismatched CD45 allele (CD45.1) that allowed separation from the CD45.2+ Het Imm CD4+ cells during flow cytometric analyses. After the 12 hour incubation, cells were harvested, stained for cell surface markers, and permeabilized for ICS using fluorescent antibodies to stain for CD45.1-PE/Cy7 (clone A20), CD25.2-BV421 (clone104), CD3ε-APC/Cy7 (clone 145-2C11), CD4-APC (clone GK1.5), IL-17A-PEeFluor610 (clone 17B7), IFN-γ-FITC (clone XMG1.2), and IL-22-PE (clone 1H8PWSR). All antibodies were from eBioscience except for the CD4-APC antibody which was from Biolegend. Cells were initially gated for live (using eFluor506; eBioscience) CD45.2+CD3ε+ cells.
Lung-resident memory cells
Left (ipsilateral) and right (contralateral) lobes of uninfected Het Imm (i.t.) and saline mice were digested separately using collagenase as described above. Single-cell suspensions were prepared for flow cytometry and labeled with the following antibodies: CD45-BV510 (clone 30-F11), CD3ε-APC/Cy7 (clone 145-2C11), CD4-PE/Cy7 (clone GK1.5), CD11a-APC (clone M17/4), CD69-PE (clone H1.2F3), CD62L-FITC (clone MEL-14), and CD44-PEeFluor610 (clone IM7) (Biolegend). Lung resident memory CD4+ T cells were defined as living cells (L/D−) that were CD45+/CD3+/CD4+/CD11ahi/CD69+/CD62Llo/CD44hi. Data were expressed as % CD11ahiCD69+ cells of CD4+ T cells.
Adoptive Transfer of Het Imm CD4+ Lymphocytes
Single-cell suspensions of lungs from uninfected Het Imm mice (CD45.2) were prepared as described above with the following modification. Prior to sacrifice, 2.5 μg of anti-CD45-APC antibody (clone 30-F11) was administered i.v. to label intra-vascular cells, as previously described20, 60. Mice were euthanized 3 minutes after i.v. antibody administration, and the lungs were cleared of blood via the right ventricle using a 24 gauge angiocather and perfusing with 10 mL of HBSS. Spleens from uninfected Het Imm mice were also harvested and single-cell suspensions were generated by passage through a 70uM filter. All cell suspensions were labeled with CD45-PE/Cy7 (clone 30-F11), CD4-PE (clone GK1.5) and a viability dye. A FACSAriaII was used to sort cells that were live, CD45+ but not labeled by the intravascular marker, and CD4+. Naïve mice received saline, lung-derived CD4+ cells, or spleen-derived CD4+ cells from Het Imm mice, transferred via i.v. tail vein injection. After 3 days, recipient mice were challenged i.t with Sp3 as described above. 24h later, left lung lobes were harvested and mRNA was extracted as previously described55. qRT-PCR for IL-17A was performed using a Taqman probe set (Mm00439618_m1) (Life Technologies)
Statistics
All statistical analyses were performed using GraphPad Prism 6.0 (GraphPad). CFU data were expressed as individual values with medians, while all other data were shown as means ± standard error of the mean (SEM). Two groups were compared using either Student’s t test or Mann-Whitney U test for non-parametric data. Multiple groups were compared using a one- or two-way analysis of variance (ANOVA) followed by a post hoc test for multiple comparisons (specified in figure legends). Multiple non-parametric data sets were compared using a Kruskal-Wallis test. Data sets included results pooled from multiple independent experiments. Differences were considered significant if P < 0.05.
Acknowledgments
This research was supported by research grants from the NIH to JPM (R01 AI115053, R01 HL068153, R01 HL079392, R35 HL135756), LJQ (R01 HL111449), and MRJ (R01 HL104053), as well as by NIH training grants (T32 HL007035, T32 AI007309).
We would like to thank Dr. Jennifer Snyder-Cappione and the Flow Cytometry Core Facility at Boston University for their advice and service and Mary Zabinski for her technical assistance.
Footnotes
Author Contributions: All authors participated in the interpretation of experiments and review of the manuscript. N.M.S.S. led the performance of all experiments, collected and analyzed data, performed statistical analyses, and drafted the manuscript. G.A.W. assisted with experimental design, experimental execution, and data analysis. F.T.C. contributed to the technical design and execution of the in vivo animal model. K.L.H. assisted with the design of the flow cytometry experiments. K.Y. contributed to the experimental design and interpretation of the results. E.L. provided technical assistance with all aspects of the project. R.M. aided in the formation of the manuscript and contributed to data analysis and interpretation. H.D. assisted with experimental design of stimulation assays and contributed to data analysis and interpretation. M.R.J and L.J.Q. contributed to the experimental design, data analysis, and interpretation. J.P.M conceptualized the line of investigation, supervised experiments, assisted in experimental design and analysis, and contributed to the drafting of the manuscript.
Disclosures: The authors declare no conflicts of interest
References
- 1.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4(3):144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 2.Mizgerd JP. Respiratory infection and the impact of pulmonary immunity on lung health and disease. Am J Respir Crit Care Med. 2012;186(9):824–829. doi: 10.1164/rccm.201206-1063PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curns AT, Holman RC, Sejvar JJ, Owings MF, Schonberger LB. Infectious disease hospitalizations among older adults in the United States from 1990 through 2002. Arch Intern Med. 2005;165(21):2514–2520. doi: 10.1001/archinte.165.21.2514. [DOI] [PubMed] [Google Scholar]
- 4.Quinton LJ, Mizgerd JP. Dynamics of lung defense in pneumonia: resistance, resilience, and remodeling. Annu Rev Physiol. 2015;77:407–430. doi: 10.1146/annurev-physiol-021014-071937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yildirim I, Shea KM, Pelton SI. Pneumococcal Disease in the Era of Pneumococcal Conjugate Vaccine. Infect Dis Clin North Am. 2015;29(4):679–697. doi: 10.1016/j.idc.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malley R. Antibody and cell-mediated immunity to Streptococcus pneumoniae: implications for vaccine development. J Mol Med (Berl) 2010;88(2):135–142. doi: 10.1007/s00109-009-0579-4. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson TM, Li CK, Chui CS, Huang AK, Perkins M, Liebner JC, et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat Med. 2012;18(2):274–280. doi: 10.1038/nm.2612. [DOI] [PubMed] [Google Scholar]
- 8.Sridhar S, Begom S, Bermingham A, Hoschler K, Adamson W, Carman W, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19(10):1305–1312. doi: 10.1038/nm.3350. [DOI] [PubMed] [Google Scholar]
- 9.McMichael AJ, Gotch FM, Noble GR, Beare PA. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309(1):13–17. doi: 10.1056/NEJM198307073090103. [DOI] [PubMed] [Google Scholar]
- 10.Lundgren A, Bhuiyan TR, Novak D, Kaim J, Reske A, Lu YJ, et al. Characterization of Th17 responses to Streptococcus pneumoniae in humans: comparisons between adults and children in a developed and a developing country. Vaccine. 2012;30(26):3897–3907. doi: 10.1016/j.vaccine.2012.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giefing C, Meinke AL, Hanner M, Henics T, Bui MD, Gelbmann D, et al. Discovery of a novel class of highly conserved vaccine antigens using genomic scale antigenic fingerprinting of pneumococcus with human antibodies. J Exp Med. 2008;205(1):117–131. doi: 10.1084/jem.20071168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wright AK, Bangert M, Gritzfeld JF, Ferreira DM, Jambo KC, Wright AD, et al. Experimental human pneumococcal carriage augments IL-17A-dependent T-cell defence of the lung. PLoS Pathog. 2013;9(3):e1003274. doi: 10.1371/journal.ppat.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strutt TM, McKinstry KK, Marshall NB, Vong AM, Dutton RW, Swain SL. Multipronged CD4(+) T-cell effector and memory responses cooperate to provide potent immunity against respiratory virus. Immunol Rev. 2013;255(1):149–164. doi: 10.1111/imr.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Jiang B, Guo Y, Li W, Tian Y, Sonnenberg GF, et al. Cross-protective mucosal immunity mediated by memory Th17 cells against Streptococcus pneumoniae lung infection. Mucosal Immunol. 2016 doi: 10.1038/mi.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mueller SN, Gebhardt T, Carbone FR, Heath WR. Memory T cell subsets, migration patterns, and tissue residence. Annu Rev Immunol. 2013;31:137–161. doi: 10.1146/annurev-immunol-032712-095954. [DOI] [PubMed] [Google Scholar]
- 16.Clark RA. Resident memory T cells in human health and disease. Sci Transl Med. 2015;7(269):269rv261. doi: 10.1126/scitranslmed.3010641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thawer SG, Horsnell WG, Darby M, Hoving JC, Dewals B, Cutler AJ, et al. Lung-resident CD4(+) T cells are sufficient for IL-4Ralpha-dependent recall immunity to Nippostrongylus brasiliensis infection. Mucosal Immunol. 2014;7(2):239–248. doi: 10.1038/mi.2013.40. [DOI] [PubMed] [Google Scholar]
- 18.Connor LM, Harvie MC, Rich FJ, Quinn KM, Brinkmann V, Le Gros G, et al. A key role for lung-resident memory lymphocytes in protective immune responses after BCG vaccination. Eur J Immunol. 2010;40(9):2482–2492. doi: 10.1002/eji.200940279. [DOI] [PubMed] [Google Scholar]
- 19.Turner DL, Bickham KL, Thome JJ, Kim CY, D’Ovidio F, Wherry EJ, et al. Lung niches for the generation and maintenance of tissue-resident memory T cells. Mucosal Immunol. 2014;7(3):501–510. doi: 10.1038/mi.2013.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrancois L, Farber DL. Cutting edge: Tissue-retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol. 2011;187(11):5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 22.Purwar R, Campbell J, Murphy G, Richards WG, Clark RA, Kupper TS. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS One. 2011;6(1):e16245. doi: 10.1371/journal.pone.0016245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJ, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity. 2013;38(1):187–197. doi: 10.1016/j.immuni.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005;175(11):7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Z, Chiou TT, Stossel TP, Kobzik L. Plasma gelsolin improves lung host defense against pneumonia by enhancing macrophage NOS3 function. Am J Physiol Lung Cell Mol Physiol. 2015;309(1):L11–16. doi: 10.1152/ajplung.00094.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kadioglu A, Weiser JN, Paton JC, Andrew PW. The role of Streptococcus pneumoniae virulence factors in host respiratory colonization and disease. Nat Rev Microbiol. 2008;6(4):288–301. doi: 10.1038/nrmicro1871. [DOI] [PubMed] [Google Scholar]
- 27.Salt P, Banner C, Oh S, Yu LM, Lewis S, Pan D, et al. Social mixing with other children during infancy enhances antibody response to a pneumococcal conjugate vaccine in early childhood. Clin Vaccine Immunol. 2007;14(5):593–599. doi: 10.1128/CVI.00344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans SE, Xu Y, Tuvim MJ, Dickey BF. Inducible innate resistance of lung epithelium to infection. Annu Rev Physiol. 2010;72:413–435. doi: 10.1146/annurev-physiol-021909-135909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, et al. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med. 2004;10(9):927–934. doi: 10.1038/nm1091. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto K, Ferrari JD, Cao Y, Ramirez MI, Jones MR, Quinton LJ, et al. Type I alveolar epithelial cells mount innate immune responses during pneumococcal pneumonia. J Immunol. 2012;189(5):2450–2459. doi: 10.4049/jimmunol.1200634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittet LA, Quinton LJ, Yamamoto K, Robson BE, Ferrari JD, Algul H, et al. Earliest innate immune responses require macrophage RelA during pneumococcal pneumonia. Am J Respir Cell Mol Biol. 2011;45(3):573–581. doi: 10.1165/rcmb.2010-0210OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aujla SJ, Dubin PJ, Kolls JK. Th17 cells and mucosal host defense. Semin Immunol. 2007;19(6):377–382. doi: 10.1016/j.smim.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, et al. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35(6):997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 35.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 36.Yamada M, Gomez JC, Chugh PE, Lowell CA, Dinauer MC, Dittmer DP, et al. Interferon-gamma production by neutrophils during bacterial pneumonia in mice. Am J Respir Crit Care Med. 2011;183(10):1391–1401. doi: 10.1164/rccm.201004-0592OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75(2):163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 38.Wilson R, Cohen JM, Jose RJ, de Vogel C, Baxendale H, Brown JS. Protection against Streptococcus pneumoniae lung infection after nasopharyngeal colonization requires both humoral and cellular immune responses. Mucosal Immunol. 2015;8(3):627–639. doi: 10.1038/mi.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malley R, Srivastava A, Lipsitch M, Thompson CM, Watkins C, Tzianabos A, et al. Antibody-independent, interleukin-17A-mediated, cross-serotype immunity to pneumococci in mice immunized intranasally with the cell wall polysaccharide. Infect Immun. 2006;74(4):2187–2195. doi: 10.1128/IAI.74.4.2187-2195.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kamei A, Coutinho-Sledge YS, Goldberg JB, Priebe GP, Pier GB. Mucosal vaccination with a multivalent, live-attenuated vaccine induces multifactorial immunity against Pseudomonas aeruginosa acute lung infection. Infect Immun. 2011;79(3):1289–1299. doi: 10.1128/IAI.01139-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hogan RJ, Zhong W, Usherwood EJ, Cookenham T, Roberts AD, Woodland DL. Protection from respiratory virus infections can be mediated by antigen-specific CD4(+) T cells that persist in the lungs. J Exp Med. 2001;193(8):981–986. doi: 10.1084/jem.193.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483(7388):227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de Roux A, Cavalcanti M, Marcos MA, Garcia E, Ewig S, Mensa J, et al. Impact of alcohol abuse in the etiology and severity of community-acquired pneumonia. Chest. 2006;129(5):1219–1225. doi: 10.1378/chest.129.5.1219. [DOI] [PubMed] [Google Scholar]
- 44.Torres A, Peetermans WE, Viegi G, Blasi F. Risk factors for community-acquired pneumonia in adults in Europe: a literature review. Thorax. 2013;68(11):1057–1065. doi: 10.1136/thoraxjnl-2013-204282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160(20):3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 46.Mizgerd JP. Community-Acquired Pneumonia Requiring Hospitalization. N Engl J Med. 2015;373(24):2380. doi: 10.1056/NEJMc1511751. [DOI] [PubMed] [Google Scholar]
- 47.Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature. 2016;532(7600):512–516. doi: 10.1038/nature17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mizgerd JP, Skerrett SJ. Animal models of human pneumonia. Am J Physiol Lung Cell Mol Physiol. 2008;294(3):L387–398. doi: 10.1152/ajplung.00330.2007. [DOI] [PubMed] [Google Scholar]
- 49.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-Acquired Pneumonia Requiring Hospitalization among U.S. Adults. N Engl J Med. 2015;373(5):415–427. doi: 10.1056/NEJMoa1500245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaid A, Mackay LK, Rahimpour A, Braun A, Veldhoen M, Carbone FR, et al. Persistence of skin-resident memory T cells within an epidermal niche. Proc Natl Acad Sci U S A. 2014;111(14):5307–5312. doi: 10.1073/pnas.1322292111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Maele L, Carnoy C, Cayet D, Ivanov S, Porte R, Deruy E, et al. Activation of Type 3 innate lymphoid cells and interleukin 22 secretion in the lungs during Streptococcus pneumoniae infection. J Infect Dis. 2014;210(3):493–503. doi: 10.1093/infdis/jiu106. [DOI] [PubMed] [Google Scholar]
- 52.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Boyman O, Sprent J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol. 2012;12(3):180–190. doi: 10.1038/nri3156. [DOI] [PubMed] [Google Scholar]
- 54.Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, et al. Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity. 2016;44(1):155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, et al. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol. 2007;178(3):1896–1903. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zens KD, Chen JK, Farber DL. Vaccine-generated lung tissue-resident memory T cells provide heterosubtypic protection to influenza infection. JCI insight. 2016;1(10) doi: 10.1172/jci.insight.85832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, et al. Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice. J Exp Med. 1997;186(8):1357–1364. doi: 10.1084/jem.186.8.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. Am J Respir Cell Mol Biol. 2008;38(6):699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu YJ, Yadav P, Clements JD, Forte S, Srivastava A, Thompson CM, et al. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin Vaccine Immunol. 2010;17(6):1005–1012. doi: 10.1128/CVI.00036-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson KG, Sung H, Skon CN, Lefrancois L, Deisinger A, Vezys V, et al. Cutting edge: intravascular staining redefines lung CD8 T cell responses. J Immunol. 2012;189(6):2702–2706. doi: 10.4049/jimmunol.1201682. [DOI] [PMC free article] [PubMed] [Google Scholar]