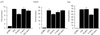Regulation of p53 function and stability by phosphorylation - PubMed (original) (raw)
Regulation of p53 function and stability by phosphorylation
M Ashcroft et al. Mol Cell Biol. 1999 Mar.
Abstract
The p53 tumor suppressor protein can be phosphorylated at several sites within the N- and C-terminal domains, and several protein kinases have been shown to phosphorylate p53 in vitro. In this study, we examined the activity of p53 proteins with combined mutations at all of the reported N-terminal phosphorylation sites (p53N-term), all of the C-terminal phosphorylation sites (p53C-term), or all of the phosphorylation sites together (p53N/C-term). Each of these mutant proteins retained transcriptional transactivation functions, indicating that phosphorylation is not essential for this activity of p53, although a subtle contribution of the C-terminal phosphorylation sites to the activation of expression of the endogenous p21(Waf1/Cip1)-encoding gene was detected. Mutation of the phosphorylation sites to alanine did not affect the sensitivity of p53 to binding to or degradation by Mdm2, although alteration of residues 15 and 37 to aspartic acid, which could mimic phosphorylation, resulted in a slight resistance to Mdm2-mediated degradation, consistent with recent reports that phosphorylation at these sites inhibits the p53-Mdm2 interaction. However, expression of the phosphorylation site mutant proteins in both wild-type p53-expressing and p53-null lines showed that all of the mutant proteins retained the ability to be stabilized following DNA damage. This indicates that phosphorylation is not essential for DNA damage-induced stabilization of p53, although phosphorylation could clearly contribute to p53 stabilization under some conditions.
Figures
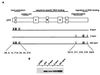
FIG. 1
(A) Schematic representation of the human p53 protein indicating the important functional domains (transactivation, proline rich, sequence-specific DNA binding, oligomerization, and regulation of DNA binding), conserved box regions I to V, and potential sites of phosphorylation within the N- and C-terminal regions. To generate N-terminal phosphorylation mutant p53 (N-term), Ser6, Ser9, Ser15, Thr18, Ser20, Ser33, and Ser37 were mutated to Ala in combination. To generate C-terminal phosphorylation mutant p53 (C-term), Ser315, Ser371, Ser376, Ser378, and Ser392 were mutated to Ala in combination. N- and C-terminal phosphorylation mutant p53 (N/C-term) was generated by a combination of the above. (B) Western blot analysis (using PAb1801) of Saos-2 cells transiently expressing wild-type p53 and the p53N-term, p53C-term, and p53N/C-term mutant proteins after transient transfection with 5 μg of the respective plasmid. Cells were harvested at 24 h.
FIG. 2
Relative phosphorylation of p53 mutant proteins in vivo. Phosphorylation site mutant p53 proteins (5 μg) were transiently transfected into Saos-2 cells. At approximately 4 h prior to harvesting (at 24 h), the cells were washed and incubated in 5 ml of phosphate-free medium (supplemented with 10% dialyzed FCS) containing 1 mCi of 32P-labeled sodium orthophosphate (per 10-cm2 dish). Cells were lysed with radioimmunoprecipitation assay lysis buffer, and p53 protein was immunoprecipitated with PAb1801-protein A Sepharose beads. (A) Phosphorylated protein was assessed by SDS–10% PAGE (top), and blots were reprobed with PAb1801 to assess immunoprecipitated p53 levels (bottom). (B) Phosphorylated protein was quantified by PhosphorImager analysis and represented graphically by using absolute integrated optical density units (I.O.D.). The graph represents the results of four independent experiments.
FIG. 3
Transcriptional activation by p53 phosphorylation mutant proteins assessed by using a luciferase reporter assay. p53 phosphorylation mutant proteins (50 ng) were cotransfected into Saos-2 cells with 5 μg of either a human p21Waf1/Cip1, Mdm2, or bax luciferase reporter construct. Five micrograms of pCB6+βgal was included to assess transfection efficiency. Cells were harvested at 24 h, lysed, and assessed for luciferase and β-galactosidase activities. Each graph shows fold activation relative to that of the pCB6+ control harvested at 24 h.
FIG. 4
Wild-type p53 and p53 phosphorylation mutant proteins (5 μg) were transiently transfected into Saos-2 cells and the cells were harvested at 24 h for Western blot analysis to assess p21Waf1/Cip1 protein levels by using an anti-p21 antibody.
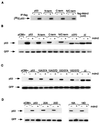
FIG. 5
(A) In vitro association assay using in vitro-translated, unlabeled, human flag-Mdm2 (cold) and 35S-labeled, in vitro-translated wild-type p53 and p53N-term, p53C-term, and p53N/C-term mutant proteins. Complexes were immunoprecipitated (IP) with anti-flag antibody in the presence of protein G Sepharose beads, and radioactively labeled p53 proteins were assessed by SDS–10% PAGE. (B) Western blot analysis (using PAb1801) of Saos-2 cells after transient cotransfection of the phosphorylation mutant p53 (3 μg), mouse Mdm2 (9 μg), and pEGFP-N1 (1 μg). Cells were harvested at 24 h. The ΔI mutant protein (deletion of conserved box I, the region containing the Mdm2 binding site), which is resistant to Mdm2-mediated degradation, and the Δ370 mutant protein (C-terminal truncation mutant protein), which is partially resistant to Mdm2-mediated degradation, were used as controls. Relative transfection efficiency was monitored by expression of cotransfected GFP. (C) Western blot analysis (using PAb1801) of Saos-2 cells after transient cotransfection of the p53-15A/37A, -15A/37D, -15D/37A, and -15D/37D combination mutant proteins (3 μg), mouse Mdm2 (9 μg), and pEGFP-N1 (1 μg). Cells were harvested at 24 h. Relative transfection efficiency was monitored by expression of cotransfected GFP. (D) Western blot analysis (using PAb1801) of Saos-2 cells after transient cotransfection of the p53-20A, -20DD, -18, and -18D combination mutant proteins (3 μg), mouse Mdm2 (9 μg), and pEGFP-N1 (1 μg). Cells were harvested at 24 h. Relative transfection efficiency was monitored by expression of cotransfected GFP.
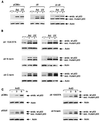
FIG. 6
(A) Schematic representation of the conserved box II deletion mutant proteins. (B) Western blot analysis (using PAb1801) of Saos-2 cells transiently expressing p53ΔII and the p53ΔII 15A/37A, p53ΔII N-term, and p53ΔII C-term mutant proteins after transient transfection with 5 μg of the respective plasmid. Cells were harvested at 24 h.
FIG. 7
Stable expression of p53ΔII combination mutant proteins in MCF-7 cells which express wild-type (wt) p53. Stable clones were isolated, and p53 protein levels were assessed by Western blot analysis using PAb1801 or D01 after treatment with either 5 nM actinomycin D (Act; harvested at 10 h) or 50-J/m2 UV (harvested at 6 h). (A) Western blot analysis of MCF-7 stable cell lines expressing either the pCB6+ control (leftmost blot), p53ΔII (middle blot), or p53ΔI-ΔII (rightmost blot). Equal protein loading was confirmed by actin expression. endo., endogenous; exo, exogenous. (B) Western blot analysis of two independent MCF-7 stable cell lines (clone 1, left; clone 2, right) expressing either p53ΔII15A/37A (top), p53ΔIIN-term (middle), or p53ΔIIC-term (bottom). Equal protein loading was confirmed by actin expression. (C) Western blot time course analysis of actinomycin D (ActD) treatment (0, 3, and 6 h) of MCF-7 cells stably expressing either pCB6+ (control), p53ΔII (clone 1), p53ΔII15A/37A (clone 1), and p53ΔIIN-term (clone 1). All blots were reprobed with an antiactin monoclonal antibody to assess protein levels. Equal protein loading was confirmed by actin expression.
FIG. 8
Stable and transient expression of p53 proteins in p53-null MEFs. (A) Western blot analysis of MEFs stably expressing either a vector control (pCB6+) or p53ΔII with or without treatment with 5 nM actinomycin (ActD). (B) Western blot analysis of MEFs transiently expressing a vector control (pCB6+) or the indicated p53 proteins without DNA damage or following treatment with actinomycin D (Act; 5 nM) or UV irradiation (50 J/m2). (C) Western blot analysis of MEFs transiently expressing a vector control (pCB6+), wild-type p53, or p53N-term without DNA damage or following treatment with actinomycin D (ActD; 5 nM) or UV irradiation (50 J/m2).
Similar articles
- The carboxy-terminal serine 392 phosphorylation site of human p53 is not required for wild-type activities.
Fiscella M, Zambrano N, Ullrich SJ, Unger T, Lin D, Cho B, Mercer WE, Anderson CW, Appella E. Fiscella M, et al. Oncogene. 1994 Nov;9(11):3249-57. Oncogene. 1994. PMID: 7936649 - Status of p53 phosphorylation and function in sensitive and resistant human cancer models exposed to platinum-based DNA damaging agents.
Mujoo K, Watanabe M, Nakamura J, Khokhar AR, Siddik ZH. Mujoo K, et al. J Cancer Res Clin Oncol. 2003 Dec;129(12):709-18. doi: 10.1007/s00432-003-0480-4. Epub 2003 Sep 26. J Cancer Res Clin Oncol. 2003. PMID: 14513366 - Binding to the naturally occurring double p53 binding site of the Mdm2 promoter alleviates the requirement for p53 C-terminal activation.
Kaku S, Iwahashi Y, Kuraishi A, Albor A, Yamagishi T, Nakaike S, Kulesz-Martin M. Kaku S, et al. Nucleic Acids Res. 2001 May 1;29(9):1989-93. doi: 10.1093/nar/29.9.1989. Nucleic Acids Res. 2001. PMID: 11328884 Free PMC article. - p53 N-terminal phosphorylation: a defining layer of complex regulation.
Jenkins LM, Durell SR, Mazur SJ, Appella E. Jenkins LM, et al. Carcinogenesis. 2012 Aug;33(8):1441-9. doi: 10.1093/carcin/bgs145. Epub 2012 Apr 12. Carcinogenesis. 2012. PMID: 22505655 Free PMC article. Review.
Cited by
- Impact of p53-associated acute myeloid leukemia hallmarks on metabolism and the immune environment.
Chomczyk M, Gazzola L, Dash S, Firmanty P, George BS, Mohanty V, Abbas HA, Baran N. Chomczyk M, et al. Front Pharmacol. 2024 Aug 5;15:1409210. doi: 10.3389/fphar.2024.1409210. eCollection 2024. Front Pharmacol. 2024. PMID: 39161899 Free PMC article. Review. - Exploring the Functional Landscape of the p53 Regulatory Domain: The Stabilizing Role of Post-Translational Modifications.
Bakker MJ, Svensson O, So Rensen HV, Skepö M. Bakker MJ, et al. J Chem Theory Comput. 2024 Jul 23;20(14):5842-5853. doi: 10.1021/acs.jctc.4c00570. Epub 2024 Jul 7. J Chem Theory Comput. 2024. PMID: 38973087 Free PMC article. - Hepatocellular Carcinoma: Old and Emerging Therapeutic Targets.
Pessino G, Scotti C, Maggi M, Immuno-Hub Consortium. Pessino G, et al. Cancers (Basel). 2024 Feb 23;16(5):901. doi: 10.3390/cancers16050901. Cancers (Basel). 2024. PMID: 38473265 Free PMC article. Review. - USP19 Negatively Regulates p53 and Promotes Cervical Cancer Progression.
Tyagi A, Karapurkar JK, Colaco JC, Sarodaya N, Antao AM, Kaushal K, Haq S, Chandrasekaran AP, Das S, Singh V, Hong SH, Suresh B, Kim KS, Ramakrishna S. Tyagi A, et al. Mol Biotechnol. 2024 Aug;66(8):2032-2045. doi: 10.1007/s12033-023-00814-y. Epub 2023 Aug 12. Mol Biotechnol. 2024. PMID: 37572221 - Pentylenetetrazole-Induced Seizures Are Increased after Kindling, Exhibiting Vitamin-Responsive Correlations to the Post-Seizures Behavior, Amino Acids Metabolism and Key Metabolic Regulators in the Rat Brain.
Aleshin VA, Graf AV, Artiukhov AV, Ksenofontov AL, Zavileyskiy LG, Maslova MV, Bunik VI. Aleshin VA, et al. Int J Mol Sci. 2023 Aug 3;24(15):12405. doi: 10.3390/ijms241512405. Int J Mol Sci. 2023. PMID: 37569781 Free PMC article.
References
- Banin S, Moyal L, Shieh S-Y, Taya Y, Anderson C W, Chessa L, Smorodinsky N I, Prives C, Reiss Y, Shiloh Y, Ziv Y. Enhanced phosphorylation of p53 by ATM in response to DNA damage. Science. 1998;281:1674–1677. - PubMed
- Banks L, Matlashewski G, Crawford L. Isolation of human p53 specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986;159:529–534. - PubMed
- Bates S, Vousden K H. p53 in signalling checkpoint arrest or apoptosis. Curr Opin Genet Dev. 1996;6:1–7. - PubMed
- Blaydes J P, Hupp T R. DNA damage triggers DRB-resistant phosphorylation of human p53 at the CK2 site. Oncogene. 1998;17:1045–1052. - PubMed
- Böttger A, Böttger V, Sparks A, W.-L. L, Howard S F, Lane D P. Design of a synthetic Mdm2 binding mini protein that activates the p53 response in vivo. Curr Biol. 1997;7:860–869. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous