Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the PTEN tumor suppressor gene - PubMed (original) (raw)
Regulation of the insulin-like developmental pathway of Caenorhabditis elegans by a homolog of the PTEN tumor suppressor gene
E B Gil et al. Proc Natl Acad Sci U S A. 1999.
Abstract
The human PTEN tumor suppressor gene is mutated in a wide variety of sporadic tumors. To determine the function of PTEN in vivo we have studied a PTEN homolog in Caenorhabditis elegans. We have generated a strong loss-of-function allele of the PTEN homolog and shown that the deficient strain is unable to enter dauer diapause. An insulin-like phosphatidylinositol 3-OH kinase (PI3'K) signaling pathway regulates dauer-stage entry. Mutations in either the daf-2 insulin receptor-like (IRL) gene or the age-1 encoded PI3'K catalytic subunit homolog cause constitutive dauer formation and also affect the life span, brood size, and metabolism of nondauer animals. Strikingly, loss-of-function mutations in the age-1 PI3'K and daf-2 IRL genes are suppressed by loss-of-function mutations in the PTEN homolog. We establish that the PTEN homolog is encoded by daf-18, a previously uncloned gene that has been shown to interact genetically with the DAF-2 IRL AGE-1 PI3'K signaling pathway. This interaction provides clear genetic evidence that PTEN acts to antagonize PI3'K function in vivo. Given the conservation of the PI3'K signaling pathway between C. elegans and mammals, the analysis of daf-18 PTEN mutant nematodes should shed light on the role of human PTEN in the etiology of metabolic disease, aging, and cancer.
Figures
Figure 1
Sequence analysis of the Caenorhabditis elegans PTEN homolog and structure of the nr2037 deletion allele. The C. elegans PTEN homolog from cosmid T07A9 is aligned with human PTEN. Identical amino acids are indicated in bold; identities and conservative changes are shaded. The phosphatase signature motif and probable catalytic aspartic acid are indicated by a box. Conserved tyrosine phosphorylation sites are indicated by asterisks. Amino acids encoded by sequences deleted in the nr2037 allele are overlined. The deletion removes bp 1,991–2,980 according to GenBank’s numbering of cosmid T07A9.
Figure 2
daf-18(e1375) consists of a complex series of duplications in the C. elegans PTEN homolog. Standard one-letter codes for both DNA and amino acid sequences are shown, with the DNA sequences in lower case. e1375 consists of a complex repeat structure in exon 4 of the daf-18 PTEN gene. bp 4995 is deleted, followed by a 13-bp duplication of bp 4,983–4,995 (corresponding to the new mutant sequence; based on GenBank’s numbering of T07A9). The first 10 bp of the repeat are duplicated again. This repeat is followed by a 6-bp subrepeat and then the insertion of an adenine. The complex repeat leads to a 6-aa insertion in exon 4, followed by a stop codon. The remainder of the exon is frameshifted with respect to the insertion. The active site of DAF-18 PTEN is indicated by an asterisk.
Figure 3
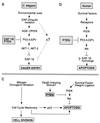
PI3′K signaling modules in mammals and nematodes have implications for cancer. (a) The C. elegans AGE-1 PI3′K homolog is recruited by the DAF-2 IRL protein. PI3′K generates PtdIns-3,4,5-P3, whereas the DAF-18 PTEN homolog degrades this second messenger. PtdIns-3,4,5-P3 and its derivative PtdIns-3,4-P2 lead to the activation of Akt-family kinases. AKT-1 and AKT-2 may directly phosphorylate the DAF-16 forkhead transcription factor, thereby antagonizing its function (17). DAF-16 forkhead activity leads to induction of the dauer state (18, 19). Loss of DAF-16 activity prevents dauer formation. (b) In mammals, PTEN and PI3′K similarly antagonize one another to regulate local PtdIns-3,4,5-P3 levels and thus Akt activation. Although C. elegans Akt homologs have not been shown to play a role in nematode programmed cell death, mammalian Akt can potently protect cells against apoptosis. DAF-16 homologs (18) of the FKHR family (38) may play a role in the regulation of apoptosis by Akt. (c) PTEN and PI3′K regulate the integration of extracellular survival signals into the normal cell division cycle. Loss of PTEN results in increased Akt activity and increased cell survival even in the presence of the proapoptotic cues induced by aberrant cell division.
Similar articles
- The PTEN tumor suppressor homolog in Caenorhabditis elegans regulates longevity and dauer formation in an insulin receptor-like signaling pathway.
Mihaylova VT, Borland CZ, Manjarrez L, Stern MJ, Sun H. Mihaylova VT, et al. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7427-32. doi: 10.1073/pnas.96.13.7427. Proc Natl Acad Sci U S A. 1999. PMID: 10377431 Free PMC article. - The C. elegans PTEN homolog, DAF-18, acts in the insulin receptor-like metabolic signaling pathway.
Ogg S, Ruvkun G. Ogg S, et al. Mol Cell. 1998 Dec;2(6):887-93. doi: 10.1016/s1097-2765(00)80303-2. Mol Cell. 1998. PMID: 9885576 - Regulation of dauer larva development in Caenorhabditis elegans by daf-18, a homologue of the tumour suppressor PTEN.
Rouault JP, Kuwabara PE, Sinilnikova OM, Duret L, Thierry-Mieg D, Billaud M. Rouault JP, et al. Curr Biol. 1999 Mar 25;9(6):329-32. doi: 10.1016/s0960-9822(99)80143-2. Curr Biol. 1999. PMID: 10209098 - Modulation of cellular apoptotic potential: contributions to oncogenesis.
Stambolic V, Mak TW, Woodgett JR. Stambolic V, et al. Oncogene. 1999 Nov 1;18(45):6094-103. doi: 10.1038/sj.onc.1203126. Oncogene. 1999. PMID: 10557100 Review. - C. elegans as a model to study PTEN's regulation and function.
Liu J, Chin-Sang ID. Liu J, et al. Methods. 2015 May;77-78:180-90. doi: 10.1016/j.ymeth.2014.12.009. Epub 2014 Dec 13. Methods. 2015. PMID: 25514044 Review.
Cited by
- Genetic redundancy masks diverse functions of the tumor suppressor gene PTEN during C. elegans development.
Suzuki Y, Han M. Suzuki Y, et al. Genes Dev. 2006 Feb 15;20(4):423-8. doi: 10.1101/gad.1378906. Genes Dev. 2006. PMID: 16481471 Free PMC article. - Time-course transcriptional profiling of human amniotic fluid-derived stem cells using microarray.
Kim YW, Kim HJ, Bae SM, Kim YJ, Shin JC, Chun HJ, Rhie JW, Kim J, Kim H, Ahn WS. Kim YW, et al. Cancer Res Treat. 2010 Jun;42(2):82-94. doi: 10.4143/crt.2010.42.2.82. Epub 2010 Jun 30. Cancer Res Treat. 2010. PMID: 20622962 Free PMC article. - The PTEN tumor suppressor homolog in Caenorhabditis elegans regulates longevity and dauer formation in an insulin receptor-like signaling pathway.
Mihaylova VT, Borland CZ, Manjarrez L, Stern MJ, Sun H. Mihaylova VT, et al. Proc Natl Acad Sci U S A. 1999 Jun 22;96(13):7427-32. doi: 10.1073/pnas.96.13.7427. Proc Natl Acad Sci U S A. 1999. PMID: 10377431 Free PMC article. - Quantitative and molecular genetic analyses of mutations increasing Drosophila life span.
Magwire MM, Yamamoto A, Carbone MA, Roshina NV, Symonenko AV, Pasyukova EG, Morozova TV, Mackay TF. Magwire MM, et al. PLoS Genet. 2010 Jul 29;6(7):e1001037. doi: 10.1371/journal.pgen.1001037. PLoS Genet. 2010. PMID: 20686706 Free PMC article. - PTEN expression causes feedback upregulation of insulin receptor substrate 2.
Simpson L, Li J, Liaw D, Hennessy I, Oliner J, Christians F, Parsons R. Simpson L, et al. Mol Cell Biol. 2001 Jun;21(12):3947-58. doi: 10.1128/MCB.21.12.3947-3958.2001. Mol Cell Biol. 2001. PMID: 11359902 Free PMC article.
References
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. - PubMed
- Steck P A, Perhouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. - PubMed
- Tashiro H, Blazes M S, Wu R, Cho K R, Bose S, Wang S I, Li J, Parsons R, Ellenson L H. Cancer Res. 1997;57:3935–3940. - PubMed
- Guldberg P, thor Straten P, Birck A, Ahrenkil V, Kirkin A F, Zeuthen J. Cancer Res. 1997;57:3660–3663. - PubMed
- Teng D F, Hu R, Lin H, Davis T, Iliev D, Frye C, Swedlund B, Hansen K L, Vinson V L, Gumpper K L, et al. Cancer Res. 1997;57:5221–5225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous