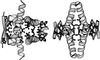Mutually compensatory mutations during evolution of the tetramerization domain of tumor suppressor p53 lead to impaired hetero-oligomerization - PubMed (original) (raw)
Mutually compensatory mutations during evolution of the tetramerization domain of tumor suppressor p53 lead to impaired hetero-oligomerization
M G Mateu et al. Proc Natl Acad Sci U S A. 1999.
Abstract
We have measured the stability and stoichiometry of variants of the human p53 tetramerization domain to assess the effects of mutation on homo- and hetero-oligomerization. The residues chosen for mutation were those in the hydrophobic core that we had previously found to be critical for its stability but are not conserved in human p73 or p51 or in p53-related proteins from invertebrates or vertebrates. The mutations introduced were either single natural mutations or combinations of mutations present in p53-like proteins from different species. Most of the mutations were substantially destabilizing when introduced singly. The introduction of multiple mutations led to two opposite effects: some combinations of mutations that have occurred during the evolution of the hydrophobic core of the domain in p53-like proteins had additive destabilizing effects, whereas other naturally occurring combinations of mutations had little or no net effect on the stability, there being mutually compensating effects of up to 9.5 kcal/mol of tetramer. The triple mutant L332V/F341L/L344I, whose hydrophobic core represents that of the chicken p53 domain, was nearly as stable as the human domain but had impaired hetero-oligomerization with it. Thus, engineering of a functional p53 variant with a reduced capacity to hetero-oligomerize with wild-type human p53 can be achieved without any impairment in the stability and subunit affinity of the engineered homo-oligomer.
Figures
Figure 1
Ribbon model of the human p53tet structure. Two different orientations are shown on the left and right sides. The 1sak coordinates (28) for the minimum tet domain (p53 residues 326–356), obtained from the Protein Data Bank, and the program
molscript
(43) were used. The residues whose side chains were found critical for stability form most of the hydrophobic core and have been depicted as ball-and-stick models.
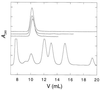
Figure 2
Analytical size-exclusion chromatography of human p53tetS and multiple mutants with the hydrophobic cores of human p51 and p73. The column was calibrated by using a set of molecular weight markers as shown in the lower part of the figure. The peaks correspond to thyroglobulin (660 kDa, _V_0), BSA (67 kDa), ovalbumin (43 kDa), chymotrypsin (25 kDa), ribonuclease A (13.7 kDa), aprotinin (6.5 kDa), and acetone (_V_t). p53tet chromatograms are shown on the upper part and have been offset for clarity. They correspond (from top to bottom) to human p53tetS and mutants L332V/M340I/F341L (core from p73), and F328L/L332V/F341L/l344I (core from p51). The initial (monomer) concentration was 40 μM.
Figure 3
(A) GdmCl denaturation (at 25°C and pH 7) of human p53tetS and some variants with natural mutations. The fraction of protein denatured is represented as a function of GdmCl concentration for wild-type tetS (□) and mutants L332V (▵), F341L (▿), L344I (⋄), and L332V/F341L/L344I (○). The protein (monomer) concentration was 40 μM. (B) Thermal denaturation (at pH 7) of human p53tetS (□) and the triple mutant L332V/341L/344I (○). The fraction of protein denatured is shown as a function of the temperature. The protein (monomer) concentration was 10 μM. For clarity, only 1 in every 10 experimental points is represented. The fitting of the experimental values to two-state transition curves by using the program Microsoft
excel
(method II in ref. 30) is indicated by solid lines.
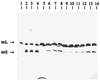
Figure 4
Analysis of hetero-oligomerization between p53tet mutants and wild-type human p53tet. The second procedure described in Materials and Methods was followed. A total of 2 nmol of His-tagged tetL were incubated either alone (lane 1) or with nontagged wild-type tetS (lane 2), mutant L332V/F341L/L344I (lane 3), or L332V/M340I/F341L (lane 4). The His-tagged homo-oligomers and the hetero-oligomers formed were separated from the remaining nontagged homo-oligomers by specific binding to Ni-NTA beads followed by elution with imidazole. Lanes 5–14, eluates containing His-tagged homo-oligomers and hetero-oligomers (if applicable) from tetL alone (lane 5) or mixtures of tetL with wild-type tetS (lanes 6–8), L332V/F341L/L344I (lanes 9–11), or L332V/M340I/F341L (lanes 12–14). TetL:tetS (wild-type or mutants) molar ratios in the corresponding incubation mixtures were 1:0.5 (lanes 6, 9, and 12), 1:1 (lanes 7, 10, and 13), or 1:2 (lanes 2–4, 8, 11, and 14). The conditions for the experiment were set up by using the alternative procedure described in Materials and Methods. In control samples with individual proteins, no His-tagged tetL was found in the washing fractions, and no untagged wild-type or mutant tet were found in the fractions eluted. Similar results consistent with those shown here were obtained in repeated experiments.
Similar articles
- Change in oligomerization specificity of the p53 tetramerization domain by hydrophobic amino acid substitutions.
Stavridi ES, Chehab NH, Caruso LC, Halazonetis TD. Stavridi ES, et al. Protein Sci. 1999 Sep;8(9):1773-9. doi: 10.1110/ps.8.9.1773. Protein Sci. 1999. PMID: 10493578 Free PMC article. - Folding of tetrameric p53: oligomerization and tumorigenic mutations induce misfolding and loss of function.
Lubin DJ, Butler JS, Loh SN. Lubin DJ, et al. J Mol Biol. 2010 Jan 29;395(4):705-16. doi: 10.1016/j.jmb.2009.11.013. Epub 2009 Nov 11. J Mol Biol. 2010. PMID: 19913028 - A meanfield approach to the thermodynamics of a protein-solvent system with application to the oligomerization of the tumor suppressor p53.
Noolandi J, Davison TS, Volkel AR, Nie X, Kay C, Arrowsmith CH. Noolandi J, et al. Proc Natl Acad Sci U S A. 2000 Aug 29;97(18):9955-60. doi: 10.1073/pnas.160075697. Proc Natl Acad Sci U S A. 2000. PMID: 10944184 Free PMC article. - The role of tetramerization in p53 function.
Chène P. Chène P. Oncogene. 2001 May 10;20(21):2611-7. doi: 10.1038/sj.onc.1204373. Oncogene. 2001. PMID: 11420672 Review.
Cited by
- Epistatic interactions modulate the evolution of mammalian mitochondrial respiratory complex components.
Azevedo L, Carneiro J, van Asch B, Moleirinho A, Pereira F, Amorim A. Azevedo L, et al. BMC Genomics. 2009 Jun 13;10:266. doi: 10.1186/1471-2164-10-266. BMC Genomics. 2009. PMID: 19523237 Free PMC article. - Structural evolution of p53, p63, and p73: implication for heterotetramer formation.
Joerger AC, Rajagopalan S, Natan E, Veprintsev DB, Robinson CV, Fersht AR. Joerger AC, et al. Proc Natl Acad Sci U S A. 2009 Oct 20;106(42):17705-10. doi: 10.1073/pnas.0905867106. Epub 2009 Oct 7. Proc Natl Acad Sci U S A. 2009. PMID: 19815500 Free PMC article. - Conformational detection of p53's oligomeric state by FlAsH Fluorescence.
Webber TM, Allen AC, Ma WK, Molloy RG, Kettelkamp CN, Dow CA, Gage MJ. Webber TM, et al. Biochem Biophys Res Commun. 2009 Jun 19;384(1):66-70. doi: 10.1016/j.bbrc.2009.04.073. Epub 2009 Apr 23. Biochem Biophys Res Commun. 2009. PMID: 19393630 Free PMC article. - Cotranslational protein assembly imposes evolutionary constraints on homomeric proteins.
Natan E, Endoh T, Haim-Vilmovsky L, Flock T, Chalancon G, Hopper JTS, Kintses B, Horvath P, Daruka L, Fekete G, Pál C, Papp B, Oszi E, Magyar Z, Marsh JA, Elcock AH, Babu MM, Robinson CV, Sugimoto N, Teichmann SA. Natan E, et al. Nat Struct Mol Biol. 2018 Mar;25(3):279-288. doi: 10.1038/s41594-018-0029-5. Epub 2018 Feb 12. Nat Struct Mol Biol. 2018. PMID: 29434345 Free PMC article.
References
- Kimura M. J Genet. 1985;64:7–19.
- Wells J A. Biochemistry. 1990;37:8509–8517. - PubMed
- Serrano L, Day A G, Fersht A R. J Mol Biol. 1993;233:305–312. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous