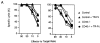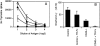TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation - PubMed (original) (raw)
TRANCE, a tumor necrosis factor family member critical for CD40 ligand-independent T helper cell activation
M F Bachmann et al. J Exp Med. 1999.
Abstract
CD40 ligand (CD40L), a tumor necrosis factor (TNF) family member, plays a critical role in antigen-specific T cell responses in vivo. CD40L expressed on activated CD4(+) T cells stimulates antigen-presenting cells such as dendritic cells, resulting in the upregulation of costimulatory molecules and the production of various inflammatory cytokines required for CD4(+) T cell priming in vivo. However, CD40L- or CD40-deficient mice challenged with viruses mount protective CD4(+) T cell responses that produce normal levels of interferon gamma, suggesting a CD40L/CD40-independent mechanism of CD4(+) T cell priming that to date has not been elucidated. Here we show that CD4(+) T cell responses to viral infection were greatly diminished in CD40-deficient mice by administration of a soluble form of TNF-related activation-induced cytokine receptor (TRANCE-R) to inhibit the function of another TNF family member, TRANCE. Thus, the TRANCE/TRANCE-R interaction provides costimulation required for efficient CD4(+) T cell priming during viral infection in the absence of CD40L/CD40. These results also indicate that not even the potent inflammatory microenvironment induced by viral infections is sufficient to elicit efficient CD4(+) T cell priming without proper costimulation provided by the TNF family (CD40L or TRANCE). Moreover, the data suggest that TRANCE/TRANCE-R may be a novel and important target for immune intervention.
Figures
Figure 1
(A) TRANCE expression is upregulated after T cell activation. Purified T cells were stimulated with anti-CD3 plus anti-CD28 and stained with TR-Fc or control hIgG1, followed by FITC-conjugated goat anti–human IgG (Fc-specific) F(ab′)2 fragment (Jackson ImmunoResearch Laboratories). (B) TRANCE induces IL-12 production in mature DCs. Mature bone marrow–derived DCs were cultured for 18 h in the presence or absence of soluble TRANCE (1 μg/ml), then fixed in 2% PFA. After incubation in 0.5% saponin, the cells were stained with anti– IL-12 p35 (C18.2), anti–IL-12 p40 (C15.1) (solid line), or control rat IgG (dotted line) followed by anti–rat IgG-PE (Jackson ImmunoResearch Laboratories), and analyzed by FACS®. In parallel experiments, soluble CD40L also induced IL-12 p35 and IL-12 p40, at levels quantitatively similar to those induced by soluble TRANCE (data not shown).
Figure 1
(A) TRANCE expression is upregulated after T cell activation. Purified T cells were stimulated with anti-CD3 plus anti-CD28 and stained with TR-Fc or control hIgG1, followed by FITC-conjugated goat anti–human IgG (Fc-specific) F(ab′)2 fragment (Jackson ImmunoResearch Laboratories). (B) TRANCE induces IL-12 production in mature DCs. Mature bone marrow–derived DCs were cultured for 18 h in the presence or absence of soluble TRANCE (1 μg/ml), then fixed in 2% PFA. After incubation in 0.5% saponin, the cells were stained with anti– IL-12 p35 (C18.2), anti–IL-12 p40 (C15.1) (solid line), or control rat IgG (dotted line) followed by anti–rat IgG-PE (Jackson ImmunoResearch Laboratories), and analyzed by FACS®. In parallel experiments, soluble CD40L also induced IL-12 p35 and IL-12 p40, at levels quantitatively similar to those induced by soluble TRANCE (data not shown).
Figure 2
(A) Blocking TRANCE does not affect isotype switching after LCMV infection. C57BL/6 mice (triangles) or CD40-deficient mice (circles) were infected with LCMV and treated with TR-Fc (filled symbols) or control hIgG1 (open symbols). LCMV-specific IgG Abs were assessed 14 d later by ELISA. One representative experiment of two is shown. (B–E) Blocking TRANCE does not affect GC formation after LCMV infection. C57BL/6 mice (B and C) and CD40-deficient mice (D and E) were infected with LCMV and treated with either TR-Fc (B and D) or control hIgG1 (C and E). The presence of GCs was assessed 14 d later in spleens by PNA staining. One representative experiment of two is shown.
Figure 2
(A) Blocking TRANCE does not affect isotype switching after LCMV infection. C57BL/6 mice (triangles) or CD40-deficient mice (circles) were infected with LCMV and treated with TR-Fc (filled symbols) or control hIgG1 (open symbols). LCMV-specific IgG Abs were assessed 14 d later by ELISA. One representative experiment of two is shown. (B–E) Blocking TRANCE does not affect GC formation after LCMV infection. C57BL/6 mice (B and C) and CD40-deficient mice (D and E) were infected with LCMV and treated with either TR-Fc (B and D) or control hIgG1 (C and E). The presence of GCs was assessed 14 d later in spleens by PNA staining. One representative experiment of two is shown.
Figure 3
Blocking TRANCE does not interfere with the induction of cytotoxic T cells but plays a role in the LCMV-specific CD4+ T cell responses. C57BL/6 mice (triangles) or CD40-deficient mice (circles) were infected with LCMV and treated with TR-Fc (filled symbols) or control hIgG1 (open symbols). (A) The presence of LCMV-specific cytotoxic T cells was assessed 8 d after infection using peptide p33– pulsed EL-4 cells as target cells. (B and C) Spleen cells were isolated 13 d later, and CD4+ T cells were purified and stimulated in vitro with LCMV-infected splenic APCs. (B) Proliferation was assessed 3 d later by [3H]thymidine incorporation. Results are shown as mean ± SEM for three mice per group. (C) Secretion of IFN-γ was assessed from culture supernatants by ELISA. Results are shown as mean ± SEM from three mice per group. Identical results were obtained with the LCMV-derived class II binding peptide 13 (data not shown). One representative experiment of two is shown.
Figure 3
Blocking TRANCE does not interfere with the induction of cytotoxic T cells but plays a role in the LCMV-specific CD4+ T cell responses. C57BL/6 mice (triangles) or CD40-deficient mice (circles) were infected with LCMV and treated with TR-Fc (filled symbols) or control hIgG1 (open symbols). (A) The presence of LCMV-specific cytotoxic T cells was assessed 8 d after infection using peptide p33– pulsed EL-4 cells as target cells. (B and C) Spleen cells were isolated 13 d later, and CD4+ T cells were purified and stimulated in vitro with LCMV-infected splenic APCs. (B) Proliferation was assessed 3 d later by [3H]thymidine incorporation. Results are shown as mean ± SEM for three mice per group. (C) Secretion of IFN-γ was assessed from culture supernatants by ELISA. Results are shown as mean ± SEM from three mice per group. Identical results were obtained with the LCMV-derived class II binding peptide 13 (data not shown). One representative experiment of two is shown.
Figure 4
LCMV-specific CD4+ T cell responses at a later time point. C57BL/6 mice (triangles) or CD40-deficient mice (circles) were infected with LCMV and treated with either TR-Fc (filled symbols) or control hIgG1 (open symbols). Spleen cells were isolated 30 d later, and CD4+ T cells were purified and stimulated in vitro with LCMV-infected splenic APCs. (A) Proliferation was assessed 3 d later by [3H]thymidine incorporation. Results are shown as mean ± SEM of triplicate values from pooled spleen cells of three mice per group. (B) Secretion of IFN-γ was assessed from culture supernatants by ELISA. Results are shown as mean ± SEM of triplicate values from pooled spleen cells of three mice per group. Identical results were obtained with the LCMV-derived class II binding peptide 13 (data not shown). One representative experiment of two is shown.
Figure 5
TRANCE plays a role in influenza virus–specific CD4+ T cell responses. CD40-deficient mice were infected with influenza virus and treated with either TR-Fc (filled circles) or control hIgG1 (open circles). Spleen cells were isolated 8 d later, and CD4+ T cells were purified and restimulated in vitro with UV light–inactivated influenza viruses. (A) Proliferation was assessed 3 d later by [3H]thymidine incorporation. Results are shown as mean ± SEM from triplicate values from pooled spleen cells of three mice per group. Background proliferation is subtracted. (B) Secretion of IFN-γ was assessed from culture supernatants by ELISA. Results are shown as mean ± SEM from three mice per group. Background is <2 U/ml. One representative experiment of three is shown.
Comment in
- TRANCE-RANK, a new signal pathway involved in lymphocyte development and T cell activation.
Green EA, Flavell RA. Green EA, et al. J Exp Med. 1999 Apr 5;189(7):1017-20. doi: 10.1084/jem.189.7.1017. J Exp Med. 1999. PMID: 10190891 Free PMC article. No abstract available.
Similar articles
- TRANCE-RANK, a new signal pathway involved in lymphocyte development and T cell activation.
Green EA, Flavell RA. Green EA, et al. J Exp Med. 1999 Apr 5;189(7):1017-20. doi: 10.1084/jem.189.7.1017. J Exp Med. 1999. PMID: 10190891 Free PMC article. No abstract available. - The role of TNF-related activation-induced cytokine-receptor activating NF-kappa B interaction in acute allograft rejection and CD40L-independent chronic allograft rejection.
Guillonneau C, Louvet C, Renaudin K, Heslan JM, Heslan M, Tesson L, Vignes C, Guillot C, Choi Y, Turka LA, Cuturi MC, Anegon I, Josien R. Guillonneau C, et al. J Immunol. 2004 Feb 1;172(3):1619-29. doi: 10.4049/jimmunol.172.3.1619. J Immunol. 2004. PMID: 14734743 - CD40-CD40 ligand interactions are critical in T-B cooperation but not for other anti-viral CD4+ T cell functions.
Oxenius A, Campbell KA, Maliszewski CR, Kishimoto T, Kikutani H, Hengartner H, Zinkernagel RM, Bachmann MF. Oxenius A, et al. J Exp Med. 1996 May 1;183(5):2209-18. doi: 10.1084/jem.183.5.2209. J Exp Med. 1996. PMID: 8642330 Free PMC article. - The role of CD40 ligand in costimulation and T-cell activation.
Grewal IS, Flavell RA. Grewal IS, et al. Immunol Rev. 1996 Oct;153:85-106. doi: 10.1111/j.1600-065x.1996.tb00921.x. Immunol Rev. 1996. PMID: 9010720 Review. - TRANCE is a TNF family member that regulates dendritic cell and osteoclast function.
Wong BR, Josien R, Choi Y. Wong BR, et al. J Leukoc Biol. 1999 Jun;65(6):715-24. doi: 10.1002/jlb.65.6.715. J Leukoc Biol. 1999. PMID: 10380891 Review.
Cited by
- Molecular mechanisms of the biphasic effects of interferon-γ on osteoclastogenesis.
Cheng J, Liu J, Shi Z, Jules J, Xu D, Luo S, Wei S, Feng X. Cheng J, et al. J Interferon Cytokine Res. 2012 Jan;32(1):34-45. doi: 10.1089/jir.2011.0019. Epub 2011 Dec 5. J Interferon Cytokine Res. 2012. PMID: 22142221 Free PMC article. - Effects of antiresorptive agents on osteomyelitis: novel insights into the pathogenesis of osteonecrosis of the jaw.
Li D, Gromov K, Proulx ST, Xie C, Li J, Crane DP, Søballe K, O'Keefe RJ, Awad HA, Xing L, Schwarz EM. Li D, et al. Ann N Y Acad Sci. 2010 Mar;1192(1):84-94. doi: 10.1111/j.1749-6632.2009.05210.x. Ann N Y Acad Sci. 2010. PMID: 20392222 Free PMC article. - Neonatal tumor necrosis factor alpha promotes diabetes in nonobese diabetic mice by CD154-independent antigen presentation to CD8(+) T cells.
Green EA, Wong FS, Eshima K, Mora C, Flavell RA. Green EA, et al. J Exp Med. 2000 Jan 17;191(2):225-38. doi: 10.1084/jem.191.2.225. J Exp Med. 2000. PMID: 10637268 Free PMC article. - CD40-independent pathways of T cell help for priming of CD8(+) cytotoxic T lymphocytes.
Lu Z, Yuan L, Zhou X, Sotomayor E, Levitsky HI, Pardoll DM. Lu Z, et al. J Exp Med. 2000 Feb 7;191(3):541-50. doi: 10.1084/jem.191.3.541. J Exp Med. 2000. PMID: 10662799 Free PMC article. - Mature dendritic cells infected with canarypox virus elicit strong anti-human immunodeficiency virus CD8+ and CD4+ T-cell responses from chronically infected individuals.
Engelmayer J, Larsson M, Lee A, Lee M, Cox WI, Steinman RM, Bhardwaj N. Engelmayer J, et al. J Virol. 2001 Mar;75(5):2142-53. doi: 10.1128/JVI.75.5.2142-2153.2001. J Virol. 2001. PMID: 11160718 Free PMC article.
References
- Foy TM, Aruffo A, Bajorah J, Buhlmann JE, Noelle RJ. Immune regulation by CD40 and its ligand. Annu Rev Immunol. 1996;14:591–617. - PubMed
- Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111–136. - PubMed
- Van Kooten C, Banchereau J. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1–77. - PubMed
- Grewal IS, Xu J, Flavell RA. Impairment of antigen-specific T-cell priming in mice lacking CD40 ligand. Nature. 1995;378:617–620. - PubMed
- van Essen D, Kikutani H, Gray D. CD40 ligand-transduced co-stimulation of T cells in the development of helper function. Nature. 1995;378:620–623. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM-07739/GM/NIGMS NIH HHS/United States
- AI-13013/AI/NIAID NIH HHS/United States
- R01 AI044264/AI/NIAID NIH HHS/United States
- AI-44264/AI/NIAID NIH HHS/United States
- T32 GM007739/GM/NIGMS NIH HHS/United States
- R01 AI013013/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials