The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells - PubMed (original) (raw)
. 1999 Apr 5;189(7):1121-8.
doi: 10.1084/jem.189.7.1121.
K Iwakabe, T Yahata, S Nishimura, A Ohta, Y Ohmi, M Sato, K Takeda, K Okumura, L Van Kaer, T Kawano, M Taniguchi, T Nishimura
Affiliations
- PMID: 10190903
- PMCID: PMC2193012
- DOI: 10.1084/jem.189.7.1121
The natural killer T (NKT) cell ligand alpha-galactosylceramide demonstrates its immunopotentiating effect by inducing interleukin (IL)-12 production by dendritic cells and IL-12 receptor expression on NKT cells
H Kitamura et al. J Exp Med. 1999.
Abstract
The natural killer T (NKT) cell ligand alpha-galactosylceramide (alpha-GalCer) exhibits profound antitumor activities in vivo that resemble interleukin (IL)-12-mediated antitumor activities. Because of these similarities between the activities of alpha-GalCer and IL-12, we investigated the involvement of IL-12 in the activation of NKT cells by alpha-GalCer. We first established, using purified subsets of various lymphocyte populations, that alpha-GalCer selectively activates NKT cells for production of interferon (IFN)-gamma. Production of IFN-gamma by NKT cells in response to alpha-GalCer required IL-12 produced by dendritic cells (DCs) and direct contact between NKT cells and DCs through CD40/CD40 ligand interactions. Moreover, alpha-GalCer strongly induced the expression of IL-12 receptor on NKT cells from wild-type but not CD1(-/-) or Valpha14(-/-) mice. This effect of alpha-GalCer required the production of IFN-gamma by NKT cells and production of IL-12 by DCs. Finally, we showed that treatment of mice with suboptimal doses of alpha-GalCer together with suboptimal doses of IL-12 resulted in strongly enhanced natural killing activity and IFN-gamma production. Collectively, these findings indicate an important role for DC-produced IL-12 in the activation of NKT cells by alpha-GalCer and suggest that NKT cells may be able to condition DCs for subsequent immune responses. Our results also suggest a novel approach for immunotherapy of cancer.
Figures
Figure 1
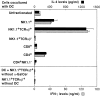
α-GalCer selectively activates NK1.1+TCR-α/β+ NKT cells. Spleen cells from C57BL/6 mice were separated into a variety of lymphoid cell subsets by cell sorting as described in Materials and Methods. Their responsiveness to α-GalCer in the presence of DCs was then determined by measuring IL-4 (▪) and IFN-γ () levels in the culture supernatants using ELISA. As a control, NK1.1+TCR-α/β+ NKT cells were cultured alone or with DCs in the absence of α-GalCer. The bars represent mean ± SE of triplicate samples.
Figure 2
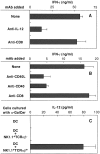
Endogenously produced IL-12 and CD40/CD40L interaction during coculture of DCs and NKT cells is essential for NKT cell activation by α-GalCer. Purified NKT cells were cocultured with DCs in the presence of α-GalCer for 36 h. The IFN-γ levels in culture supernatants were then determined by ELISA. (A) The ability of anti–IL-12 mAb to block NKT cell activation by α-GalCer. Anti-CD8 mAb was used as control rat IgG Ab. (B) The ability of anti-CD40 mAb and anti-CD40L mAb to block NKT cell activation by α-GalCer. As a control, rat anti-CD8 IgG mAb was added to the culture. (C) IL-12 production by DCs cultured with α-GalCer and NKT cells. DCs (5 × 105) were activated with 50 ng/ml of α-GalCer for 8 h in the presence or absence of NK1.1+TCR-α/β− NK cells (105) or NK1.1+TCR-α/β+ NKT cells (105). The bars represent mean ± SE of triplicate samples.
Figure 3
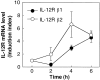
Upregulation of IL-12R expression in the spleen upon in vivo administration of α-GalCer. C57BL/6 mice were injected intravenously with α-GalCer. Various times (0, 2, 4, and 6 h) after the treatment, spleen cells were prepared and their expression of IL-12Rβ1 (•) and IL-12Rβ2 (○) mRNA was measured by quantitative RT-PCR. The IL-12R mRNA levels are represented as an induction index, as described in Materials and Methods. The bars represent mean ± SE of triplicate samples.
Figure 4
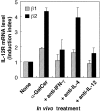
Role of IL-12 and IFN-γ in the induction of IL-12R expression in the spleen. C57BL/6 mice were injected intravenously with α-GalCer. 4 h after the injection, spleen cells were prepared and their expression of IL-12Rβ1 () and IL-12Rβ2 (▪) mRNA was measured by quantitative RT-PCR. The IL-12R mRNA levels are presented as an induction index, as described in Materials and Methods. The blocking effect of anti–IL-12 mAb and anti–IFN-γ mAb was determined by injection of these mAbs (500 μg/mouse i.p.) into the mice 1 and 0 d before the treatment with α-GalCer. The bars represent mean ± SE of triplicate samples.
Figure 5
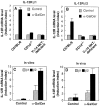
α-GalCer induces IL-12R expression on NKT cells. C57BL/6 wild-type mice, CD1d−/− mice, and NKT-deficient mice were injected intravenously with vehicle () or α-GalCer (▪). 4 h after the injection, spleen cells were prepared and their expression of IL-12Rβ1 (A) and IL-12Rβ2 (B) mRNA was measured by quantitative RT-PCR. (C) Spleen cells from wild-type mice were cultured with DCs plus α-GalCer or vehicle for 8 h, NK1.1+TCR-α/β+ NKT cells were purified by cell sorting, and the expression of IL-12Rβ1 () and IL-12Rβ2 (▪) was examined by quantitative RT-PCR. (D) Wild-type mice were injected intravenously with α-GalCer or vehicle; after 6 h the spleens from these mice were isolated, and NK1.1+TCR-α/β+ NKT cells were purified by cell sorting, and expression of IL-12Rβ1 () and IL-12Rβ2 (▪) was examined by quantitative RT-PCR. The IL-12R mRNA levels are presented as an induction index, as described in Materials and Methods. The bars represent mean ± SE of triplicate samples.
Figure 6
Synergistic effect of α-GalCer and IL-12 in vivo. C57BL/6 mice were injected with a suboptimal dose of α-GalCer (200 ng/mouse i.v.) and 6 h later, mice were injected with a suboptimal dose of IL-12 (200 U/mouse i.p.). 1 d after the treatment with IL-12, the mice were killed and splenic natural killing activity (A) and serum IFN-γ levels (B) were determined as described in Materials and Methods. The bars represent mean ± SE of triplicate samples.
Similar articles
- A novel function of Valpha14+CD4+NKT cells: stimulation of IL-12 production by antigen-presenting cells in the innate immune system.
Tomura M, Yu WG, Ahn HJ, Yamashita M, Yang YF, Ono S, Hamaoka T, Kawano T, Taniguchi M, Koezuka Y, Fujiwara H. Tomura M, et al. J Immunol. 1999 Jul 1;163(1):93-101. J Immunol. 1999. PMID: 10384104 - Natural killer T cells from interleukin-4-deficient mice are defective in early interferon-gamma production in response to alpha-galactosylceramide.
Togashi Y, Chamoto K, Wakita D, Tsutsumi N, Iwakura Y, Matsubara N, Kitamura H, Nishimura T. Togashi Y, et al. Cancer Sci. 2007 May;98(5):721-5. doi: 10.1111/j.1349-7006.2007.00451.x. Epub 2007 Mar 14. Cancer Sci. 2007. PMID: 17359285 Free PMC article. - Differential regulation of Th1 and Th2 functions of NKT cells by CD28 and CD40 costimulatory pathways.
Hayakawa Y, Takeda K, Yagita H, Van Kaer L, Saiki I, Okumura K. Hayakawa Y, et al. J Immunol. 2001 May 15;166(10):6012-8. doi: 10.4049/jimmunol.166.10.6012. J Immunol. 2001. PMID: 11342617 - Innate Valpha14(+) natural killer T cells mature dendritic cells, leading to strong adaptive immunity.
Fujii S, Shimizu K, Hemmi H, Steinman RM. Fujii S, et al. Immunol Rev. 2007 Dec;220:183-98. doi: 10.1111/j.1600-065X.2007.00561.x. Immunol Rev. 2007. PMID: 17979847 Review. - Role of alpha-galactosylceramide-activated Valpha14 natural killer T cells in the regulation of allergic diseases.
Iwamura C, Nakayama T. Iwamura C, et al. Allergol Int. 2007 Mar;56(1):1-6. doi: 10.2332/allergolint.R-06-136. Epub 2007 Jan 29. Allergol Int. 2007. PMID: 17259803 Review.
Cited by
- α-Galactosylceramide-activated murine NK1.1(+) invariant-NKT cells in the myometrium induce miscarriages in mice.
Ichikawa T, Negishi Y, Shimizu M, Takeshita T, Takahashi H. Ichikawa T, et al. Eur J Immunol. 2016 Aug;46(8):1867-77. doi: 10.1002/eji.201545923. Epub 2016 Jun 8. Eur J Immunol. 2016. PMID: 27198610 Free PMC article. - Natural killer T cell ligand alpha-galactosylceramide inhibited lymph node metastasis of highly metastatic melanoma cells.
Nakui M, Iwakabe K, Ohta A, Sekimoto M, Sato M, Makuuchi H, Kawano T, Taniguchi M, Nishimura T. Nakui M, et al. Jpn J Cancer Res. 1999 Aug;90(8):801-4. doi: 10.1111/j.1349-7006.1999.tb00818.x. Jpn J Cancer Res. 1999. PMID: 10543249 Free PMC article. - Role of CD1d-restricted NKT cells in microbial immunity.
Sköld M, Behar SM. Sköld M, et al. Infect Immun. 2003 Oct;71(10):5447-55. doi: 10.1128/IAI.71.10.5447-5455.2003. Infect Immun. 2003. PMID: 14500461 Free PMC article. Review. No abstract available. - The clinical implication and molecular mechanism of preferential IL-4 production by modified glycolipid-stimulated NKT cells.
Oki S, Chiba A, Yamamura T, Miyake S. Oki S, et al. J Clin Invest. 2004 Jun;113(11):1631-40. doi: 10.1172/JCI20862. J Clin Invest. 2004. PMID: 15173890 Free PMC article. - Valpha14 NKT cell-mediated anti-tumor responses and their clinical application.
Seino K, Fujii S, Harada M, Motohashi S, Nakayama T, Fujisawa T, Taniguchi M. Seino K, et al. Springer Semin Immunopathol. 2005 Jun;27(1):65-74. doi: 10.1007/s00281-004-0194-y. Epub 2005 Jan 14. Springer Semin Immunopathol. 2005. PMID: 15650847 Review.
References
- Fowlkes BJ, Kruisbeek AM, Ton-That H, Weston MA, Coligan JE, Schwartz RH, Pardoll DM. A novel population of T-cell receptor αβ-bearing thymocytes which predominantly express a single Vβ gene family. Nature. 1987;329:251–254. - PubMed
- Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. - PubMed
- Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol Res. 1995;7:529–534. - PubMed
- Kazuhiko M, Maeda K, Ueno H, Kobayashi E, Uchida T, Fukushima H, Koezuka Y. Antitumor activities of combined treatment with a novel immunomodulator, (2S,3S,4R)-1-O-(α-d-galactopyranosyl)-2-(N-hexacosanoylamino)-1,3,4-octadecanetriol (KRN7000), and radiotherapy in tumor-bearing mice. Oncol Res. 1996;8:155–162. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials