A Rab2 mutant with impaired GTPase activity stimulates vesicle formation from pre-Golgi intermediates - PubMed (original) (raw)
A Rab2 mutant with impaired GTPase activity stimulates vesicle formation from pre-Golgi intermediates
E J Tisdale. Mol Biol Cell. 1999 Jun.
Free PMC article
Abstract
Rab2 immunolocalizes to pre-Golgi intermediates (vesicular-tubular clusters [VTCs]) that are the first site of segregation of anterograde- and retrograde-transported proteins and a major peripheral site for COPI recruitment. Our previous work showed that Rab2 Q65L (equivalent to Ras Q61L) inhibited endoplasmic reticulum (ER)-to-Golgi transport in vivo. In this study, the biochemical properties of Rab2 Q65L were analyzed. The mutant protein binds GDP and GTP and has a low GTP hydrolysis rate that suggests that Rab2 Q65L is predominantly in the GTP-bound-activated form. The purified protein arrests vesicular stomatitis virus glycoprotein transport from VTCs in an assay that reconstitutes ER-to-Golgi traffic. A quantitative binding assay was used to measure membrane binding of beta-COP when incubated with the mutant. Unlike Rab2 that stimulates recruitment, Rab2 Q65L showed a dose-dependent decrease in membrane-associated beta-COP when incubated with rapidly sedimenting membranes (ER, pre-Golgi, and Golgi). The mutant protein does not interfere with beta-COP binding but stimulates the release of slowly sedimenting vesicles containing Rab2, beta-COP, and p53/gp58 but lacking anterograde grade-directed cargo. To complement the biochemical results, we observed in a morphological assay that Rab2 Q65L caused vesiculation of VTCs that accumulated at 15 degrees C. These data suggest that the Rab2 protein plays a role in the low-temperature-sensitive step that regulates membrane flow from VTCs to the Golgi complex and back to the ER.
Figures
Figure 1
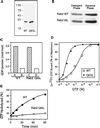
Guanine nucleotide–binding properties of bacterially produced Rab2 proteins. (A) Rab2 wild type and Rab2 Q65L were purified after expression in E. coli using ion exchange chromatography and gel filtration. The proteins were analyzed by separation on SDS-PAGE and Coomassie blue staining, and then the gel was scanned on a densitometer. The recombinant proteins were found to be ∼90% pure. (B) The purified proteins (0.5 μg) were prenylated in an in vitro reaction and then subjected to phase partitioning in Triton X-114, and the distribution of the Rab2 proteins was analyzed by SDS-PAGE and immunoblotting. The prenylation efficiency is ∼40–45% as indicated by partitioning with the detergent-rich phase. (C) Rab2 and Rab2 Q65L (10 pmol) were incubated for 1 h with 2.5 μM [3H]GDP (∼500 cpm/pmol) in the presence of 5 mM EDTA and 4.5 mM MgCl2 (bars A and C) or 10 mM MgCl2 (bars B and D), and the amount of protein-bound [3H]GDP was determined as described in MATERIALS AND METHODS. The recombinant proteins are ∼70–75% active based on their ability to bind and exchange GDP. (D) Equilibrium binding of [α-32P]GTP by Rab2 wild type and mutant is shown. Rab2 proteins (10 pmol) were incubated for 60 min at 30°C with increasing concentrations of [α-32P]GTP (1 × 10−9 to 1 × 10−4 M). Protein-bound [α-32P]GTP was captured on nitrocellulose membranes and then quantitated by liquid scintillation counting as described in MATERIALS AND METHODS. The results are normalized to the amount of binding at the highest concentration of [α-32P]GTP for each protein. (E) GTPase hydrolytic activity was measured by in-cubating Rab2 and Rab2 Q65L with [α-32P]GTP at 37°C. At the times indicated, the reactions were terminated with the addition of ice-cold 0.5 M EDTA, and the [32P]-labeled nucleotides bound to the proteins were analyzed by TLC. Results shown in all panels are representative of three independent protein purifications.
Figure 2
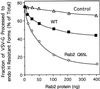
Rab2 Q65L inhibits ER-to-Golgi transport in vitro. Semi-intact NRK cells were preincubated with the indicated amount of Rab2 (WT), Rab2 Q65L, or mock-prenylated control (lacking recombinant Rab2 protein) for 30 min on ice in a transport mixture as described in MATERIALS AND METHODS. The cells were then transferred to 32°C and incubated for a total of 90 min. The fraction of VSV-G processed to the endo H–resistant forms (percent of total) was determined after analysis by SDS-PAGE and fluorography. Rab2 Q65L inhibited transport in a dose-dependent manner. Results shown are representative of three independent protein purifications.
Figure 3
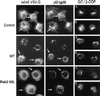
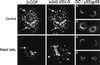
Cells incubated with Rab2 Q65L fail to transport ts045 VSV-G to the Golgi complex. NRK cells grown on coverslips were infected with ts045 VSV-G for 3 h at 39.5°C to retain ts045 VSV-G in the endoplasmic reticulum. The cells were rapidly shifted to ice, permeabilized with digitonin, and then incubated in a complete transport cocktail in the absence (Control) or presence of 100 ng of Rab2 (WT) or 100 ng of Rab2 Q65L for 40 min at 32°C. The distribution of ts045 VSV-G (left), p53/gp58 (middle), β-COP (B), and Golgi (A) staining was determined as described in MATERIALS AND METHODS. Arrows denote the alignment of costained cells. GC, Golgi complex.
Figure 4
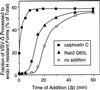
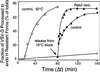
The inhibitory activity of Rab2 Q65L is downstream from the calphostin C–sensitive step. Semi-intact NRK cells were incubated at 32°C in a transport mixture described in MATERIALS AND METHODS. At the indicated time (Δt), the control (○) was transferred to ice, or 120 nM calphostin C (▪) or 100 ng of Rab2 Q65L (▴) was added and incubated with the cells for a total of 60 min. Transport was terminated by transfer of the cells to ice, and the fraction of VSV-G processed to endo H–resistant forms was determined as described in MATERIALS AND METHODS. Results shown are representative of three independent protein purifications.
Figure 5
VSV-G is rapidly transported through the Rab2 Q65L–sensitive step after accumulation at 15°C. Semi-intact NRK cells were either incubated in a transport mixture for the indicated time at 32°C (□) or preincubated at a reduced temperature (15°C) for 80 min to accumulate VSV-G in pre-Golgi VTCs. Cells preincubated at 15°C were maintained at 15°C (○), shifted to 32°C and incubated for the indicated time (Δt) before transfer to ice (▪), or supplemented with 100 ng of Rab2 Q65L and incubated for a total of 80 min (▴). The fraction of VSV-G processed to endo H–resistant forms was determined as described in METHODS AND MATERIALS. Results shown are representative of three independent protein purifications
Figure 6
Rab2 Q65L stimulates vesicle release from microsomes. (A and B) Microsomes were prepared from either NRK cell homogenates, ts045 VSV-G–infected NRK cells, or NRK cells transfected with c-myc NAGT I (Nilsson et al., 1993) or transfected with CD8 as described in MATERIALS AND METHODS and then were preincubated with 50 or 100 ng of Rab2 Q65L for 10 min on ice. Control membranes were preincubated with an equal volume of the mock-prenylated fraction (comparable with 100 ng of Rab2 Q65L), obtained as described in MATERIALS AND METHODS. Cytosol and GTPγS were added, and the incubations transferred to 37°C for 10 min to promote recruitment of soluble factors. Microsomes were collected by centrifugation (20,000 × g for 10 min) to obtain a pellet (P1). The supernatant was recentrifuged at 100,000 × g for 30 min, and the resulting pellet (P2) and P1 were separated by SDS-PAGE and immunoblotted with the respective antibodies. The blot was developed with enhanced chemiluminescence and then quantitated by densitometry. (C) Microsomes prepared from NRK cell homogenates were preincubated with 300 or 400 ng of Rab2 for 10 min on ice. Cytosol and GTPγS were added, and the incubations were then transferred to 37°C for 10 min and processed as described above. (D) P2 from control membranes incubated with GTPγS (▵), P2 from Rab2 Q65L–treated membranes incubated with GTPγS (●), or P2 from Rab2 Q65L–treated membranes incubated without GTPγS (□) was subjected to equilibrium density centrifugation as described in MATERIALS AND METHODS. The gradient was fractionated from the bottom into 300-μl fractions, and the recovered fractions were pelleted by ultracentrifugation (100,000 rpm for 30 min at 4°C) and then separated by SDS-PAGE and immunoblotted for β-COP. (E) Microsomes were preincubated in buffer with or without 200 μM BFA for 15 min on ice and then supplemented with 100 ng of Rab2 Q65L, GTPγS, and rat liver cytosol and incubated at 37°C for 10 min. The membranes were collected as described above. P1 and P2 were separated by SDS-PAGE, transferred to nitrocellulose, and then probed with an affinity-purified antibody to β-COP. Bar A, P1 from membranes incubated with Rab2 Q65L; bar B, P2 from membranes incubated with Rab2 Q65L; bar C, P1 from membranes coincubated with BFA and Rab2 Q65L; and bar D, P2 from membranes coincubated with BFA and Rab2 Q65L.
Figure 7
Peripheral VTCs containing β-COP become undetectable after incubation with Rab2 Q65L. ts045 VSV-G–infected NRK cells were incubated for 80 min at 15°C to accumulate VSV-G in VTCs. The cells were then digitonin permeabilized and incubated in transport buffer in the absence or presence of 100 ng of Rab2 Q65L for 15 min at 15°C. The distribution of β-COP (left), ts045 VSV-G (middle), p53/gp58 (B), and Golgi (A) staining was determined as described in MATERIALS AND METHODS. Arrows denote the alignment of costained cells. Arrowheads indicate peripheral VTCs.
Similar articles
- Glyceraldehyde-3-phosphate dehydrogenase is required for vesicular transport in the early secretory pathway.
Tisdale EJ. Tisdale EJ. J Biol Chem. 2001 Jan 26;276(4):2480-6. doi: 10.1074/jbc.M007567200. Epub 2000 Oct 16. J Biol Chem. 2001. PMID: 11035021 - Rab2 protein enhances coatomer recruitment to pre-Golgi intermediates.
Tisdale EJ, Jackson MR. Tisdale EJ, et al. J Biol Chem. 1998 Jul 3;273(27):17269-77. doi: 10.1074/jbc.273.27.17269. J Biol Chem. 1998. PMID: 9642298 - The role of ADP-ribosylation factor and SAR1 in vesicular trafficking in plants.
Memon AR. Memon AR. Biochim Biophys Acta. 2004 Jul 1;1664(1):9-30. doi: 10.1016/j.bbamem.2004.04.005. Biochim Biophys Acta. 2004. PMID: 15238254 Review. - Protein sorting by directed maturation of Golgi compartments.
Allan BB, Balch WE. Allan BB, et al. Science. 1999 Jul 2;285(5424):63-6. doi: 10.1126/science.285.5424.63. Science. 1999. PMID: 10390362 Review.
Cited by
- AtRabD2b and AtRabD2c have overlapping functions in pollen development and pollen tube growth.
Peng J, Ilarslan H, Wurtele ES, Bassham DC. Peng J, et al. BMC Plant Biol. 2011 Jan 26;11:25. doi: 10.1186/1471-2229-11-25. BMC Plant Biol. 2011. PMID: 21269510 Free PMC article. - Rab2 utilizes glyceraldehyde-3-phosphate dehydrogenase and protein kinase C{iota} to associate with microtubules and to recruit dynein.
Tisdale EJ, Azizi F, Artalejo CR. Tisdale EJ, et al. J Biol Chem. 2009 Feb 27;284(9):5876-84. doi: 10.1074/jbc.M807756200. Epub 2008 Dec 23. J Biol Chem. 2009. PMID: 19106097 Free PMC article. - α-Synuclein impairs macroautophagy: implications for Parkinson's disease.
Winslow AR, Chen CW, Corrochano S, Acevedo-Arozena A, Gordon DE, Peden AA, Lichtenberg M, Menzies FM, Ravikumar B, Imarisio S, Brown S, O'Kane CJ, Rubinsztein DC. Winslow AR, et al. J Cell Biol. 2010 Sep 20;190(6):1023-37. doi: 10.1083/jcb.201003122. J Cell Biol. 2010. PMID: 20855506 Free PMC article. - GAPDH binds Akt to facilitate cargo transport in the early secretory pathway.
Tisdale EJ, Talati NK, Artalejo CR, Shisheva A. Tisdale EJ, et al. Exp Cell Res. 2016 Dec 10;349(2):310-319. doi: 10.1016/j.yexcr.2016.10.025. Epub 2016 Nov 3. Exp Cell Res. 2016. PMID: 27818247 Free PMC article. - Proteome analysis of Ehrlichia chaffeensis containing phagosome membranes revealed the presence of numerous bacterial and host proteins.
Kondethimmanahalli C, Ganta RR. Kondethimmanahalli C, et al. Front Cell Infect Microbiol. 2022 Dec 23;12:1070356. doi: 10.3389/fcimb.2022.1070356. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 36619760 Free PMC article.
References
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. - PubMed
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. - PubMed
- Beckers CJM, Keller DS, Balch WE. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987;50:523–534. - PubMed
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981;256:1604–1607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous