Activating phosphorylation of the Kin28p subunit of yeast TFIIH by Cak1p - PubMed (original) (raw)
Activating phosphorylation of the Kin28p subunit of yeast TFIIH by Cak1p
J Kimmelman et al. Mol Cell Biol. 1999 Jul.
Abstract
Cyclin-dependent kinase (CDK)-activating kinases (CAKs) carry out essential activating phosphorylations of CDKs such as Cdc2 and Cdk2. The catalytic subunit of mammalian CAK, MO15/Cdk7, also functions as a subunit of the general transcription factor TFIIH. However, these functions are split in budding yeast, where Kin28p functions as the kinase subunit of TFIIH and Cak1p functions as a CAK. We show that Kin28p, which is itself a CDK, also contains a site of activating phosphorylation on Thr-162. The kinase activity of a T162A mutant of Kin28p is reduced by approximately 75 to 80% compared to that of wild-type Kin28p. Moreover, cells containing kin28(T162A) and a conditional allele of TFB3 (the ortholog of the mammalian MAT1 protein, an assembly factor for MO15 and cyclin H) are severely compromised and display a significant further reduction in Kin28p activity. This finding provides in vivo support for the previous biochemical observation that MO15-cyclin H complexes can be activated either by activating phosphorylation of MO15 or by binding to MAT1. Finally, we show that Kin28p is no longer phosphorylated on Thr-162 following inactivation of Cak1p in vivo, that Cak1p can phosphorylate Kin28p on Thr-162 in vitro, and that this phosphorylation stimulates the CTD kinase activity of Kin28p. Thus, Kin28p joins Cdc28p, the major cell cycle Cdk in budding yeast, as a physiological Cak1p substrate. These findings indicate that although MO15 and Cak1p constitute different forms of CAK, both control the cell cycle and the phosphorylation of the C-terminal domain of the large subunit of RNA polymerase II by TFIIH.
Figures
FIG. 1
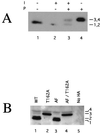
Kin28p is a phosphoprotein. (A) Phosphatase treatment. Kin28p-HA was immunoprecipitated from native yeast lysates and incubated with lambda phosphatase (P) and/or phosphatase inhibitor (I). Samples were subject to SDS-PAGE followed by immunoblotting. (B) Electrophoretic mobilities of Kin28p point mutants. Strains carrying a kin28 deletion covered by plasmids containing HA epitope-tagged Kin28p point mutants were harvested during exponential growth, lysed directly into SDS-PAGE sample buffer, and analyzed by immunoblotting. AF denotes a T18A/Y19F double mutant. “No HA” indicates that a strain lacks HA-tagged proteins. Immunoblots produced four Kin28p species (see also Fig. 2A). WT, wild type
FIG. 2
2-D gel analysis of Kin28p. (A) Protein samples containing Kin28p alleles were subjected to isoelectric focusing with a 1:1 mixture of pH 4 to 8 and pH 5 to 7 carrier ampholytes, run on SDS–12.5% polyacrylamide gels in the second dimension, and immunoblotted. To aid in identifying spots, lanes containing Kin28p and Kin28pT162A samples were run alongside tube gels in the second dimension (a). Kin28p alleles tested included wild-type (WT) Kin28p (b), Kin28pT162A (c), phosphatase (P’ase)-treated wild-type Kin28p (d), and phosphatase-treated Kin28pT162A (e). (B) To rule out the possibility of a cross-reacting species in these immunoblots, extracts from cells expressing tagged (a) or untagged (b) Kin28p were subjected to isoelectric focusing with pH 4 to 8 carrier ampholytes and processed as described for panel A.
FIG. 3
CTD kinase activity of Kin28p mutants. Kin28p was immunoprecipitated from extracts of strains expressing wild-type (WT) Kin28p or the indicated point mutants and assayed for CTD kinase activity. The portion of the gel containing the CTD peptide was processed for autoradiography (upper panel), while the portion containing Kin28p was immunoblotted with anti-HA antibodies (lower panel).
FIG. 4
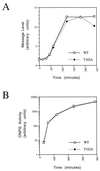
Thr-162 phosphorylation of Kin28p is not essential for transcriptional induction. (A) Osmotic stress. Cells containing KIN28 (wild type [WT]) or kin28T162A (strains YGK26 and YGK42, respectively) were inoculated into YPD containing 0.9 M NaCl and maintained. RNA was extracted at various times after induction, and transcripts were analyzed by Northern blotting with a probe derived from GPD1. Signal intensities were quantitated by phosphorimaging. (B) Galactose induction. KIN28 or kin28T162A cells containing the galactose-inducible lacZ reporter plasmid pAF21 (strains YJK1747 and YJK1749) and growing in raffinose-containing medium were collected at various times following induction of the galactose promoter by addition of 30% galactose to a final concentration of 2%. β-Galactosidase activity was measured with _o_-nitrophenyl-β-
d
-galactopyranoside (ONPG) as a substrate.
FIG. 5
Phenotype of a kin28T162A strain. (A) _kin28_Δ cells containing a _URA3_-marked wild-type (WT) KIN28 plasmid (pJK1) and _TRP1_-marked plasmids containing either KIN28 (pGK13), kin28T162A (pGK36), kin28-ts16 (pGK33), kin28D147N (pMS454), or kin28-tsT162A (pJK25) were plated on FOA-containing plates to select for loss of the URA3 marked plasmid. (B) _kin28_Δ cells containing KIN28 (YJK1869, YJK1767, and YJK1768) or kin28T162A (YJK1870, YJK1755, and YJK1756) and either an empty vector (YCplac22), kin28D147N on a low-copy-number plasmid (pMS454), or kin28D147N on a high-copy-number plasmid (pMS456) were plated on CM-Trp and grown at 37°C.
FIG. 6
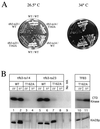
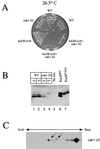
Genetic interaction between kin28ST162A and tfb3-ts. (A) Strains containing TFB3 or one of two temperature-sensitive alleles of tfb3 and either KIN28 or kin28T162A were plated and grown at 26.5 and 34°C. Cells containing kin28T162A alone showed no growth defect even at 37°C (data not shown). (B) The strains described for panel A as well as a kin28T162A strain were grown at either 23 or 37°C for 4 h. Extracts were prepared, and Kin28p immunoprecipitates were assayed for CTD kinase activity (top panel) or immunoblotted for Kin28p (bottom panel). The apparently greater Kin28pT162A activity seen in this figure compared to Fig. 3 reflects the longer exposure used in this experiment; Kin28pT162A activity is still reduced by 75 to 80% compared to that of wild-type (WT) Kin28p.
FIG. 7
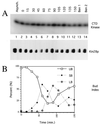
Kin28p level, activity, and phosphorylation state are constant during the cell cycle. (A) Exponentially growing KIN28 cells (YJK1773) were arrested with α-factor, washed, and released into fresh medium at 30°C. Extracts were prepared at 15-min intervals, and Kin28p immunoprecipitates were assayed for CTD kinase activity (top panel) or immunoblotted for Kin28p (bottom panel). Extracts of asynchronous cells (Asynch.) and cells arrested in mitosis with benomyl for 3.75 h (Ben 1) and 4.75 h (Ben 2) were also analyzed. (B) Bud indices of the cultures in panel A. UB, SB, and LB, unbudded, small-budded, and large-budded cells, respectively.
FIG. 8
CAK1 regulates the phosphorylation of Kin28p. (A) Genetic interaction between cak1-22 and kin28-ts16. The indicated wild-type (WT) and mutant strains (clockwise from top, YMW2, SY162, YJK1599, YJK1600, YGK24, and SY143) were plated and incubated at 26.5°C. Two isolates of wild-type and double-mutant strains are shown. (B) Kin28p is hypophosphorylated in a cak1-22 strain. Extracts were prepared from CAK1 and cak1-22 strains (YJK1610 and YJK1614, respectively) grown at 23 or 37°C for 6 h, and Kin28p immunoprecipitates were immunoblotted. (C) Extracts of a cak1-22 strain (YJK1625) grown at 37°C for 6 h were analyzed by 2-D gel electrophoresis as described for Fig. 2. Spots 3 and 4 correspond to the same Kin28p spots in Fig. 2.
FIG. 9
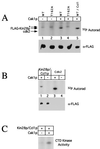
Cak1p phosphorylates Kin28p on Thr-162 in vitro. (A) Purified Cak1p was incubated with FLAG-Kin28p (lane 3), FLAG-Kin28pT162A (lane 4), or FLAG-Kin28pD147A–Ccl1p complexes (lane 5) in the presence of [γ-32P]ATP. As controls for autophosphorylation, FLAG-Kin28p and FLAG-Kin28pT162A were incubated in the absence of Cak1p (lanes 1 and 2). Phosphorylated proteins were detected by autoradiography (Autorad) following SDS-PAGE (upper panel). Note that the monomeric Kin28p samples (lanes 1 to 4) were only ∼1 to 5% pure (estimated by gel staining), resulting in significant background phosphorylation; the asterisk denotes a nonspecific species. The relative Kin28p levels in the kinase assays were determined by immunoblotting with antibodies to the FLAG tag (lower panel). WT, wild type. (B) FLAG-Kin28pD147A–Ccl1p complexes (lanes 1 and 2) or Cdk2 (lanes 3 and 4) was incubated with (lanes 1 and 3) or without (lanes 2 and 4) purified Cak1p in the presence of [γ-32P]ATP. Phosphorylated proteins were detected by autoradiography following SDS-PAGE (upper panel), and the relative Kin28p levels in the kinase assays were determined by immunoblotting with antibodies to the FLAG tag (lower panel). Cdk2 is not visible because it is not FLAG tagged. (C) Phosphorylation of Kin28p by Cak1p increases its CTD kinase activity. FLAG-Kin28p–Ccl1p complexes were incubated with (lane 2) or without (lane 1) purified Cak1p and assayed for CTD kinase activity. Phosphorimager quantitation showed that the CTD kinase activity of Kin28p increased sevenfold following incubation with Cak1p.
FIG. 10
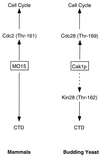
Dual regulation of transcription and the cell division cycle by CAKs. Both mammalian CAK (MO15) and yeast Cak1p affect transcription (via CTD phosphorylation) and cell division (via CDK phosphorylation). The dotted line denotes a nonessential phosphorylation.
Similar articles
- Human and yeast cdk-activating kinases (CAKs) display distinct substrate specificities.
Kaldis P, Russo AA, Chou HS, Pavletich NP, Solomon MJ. Kaldis P, et al. Mol Biol Cell. 1998 Sep;9(9):2545-60. doi: 10.1091/mbc.9.9.2545. Mol Biol Cell. 1998. PMID: 9725911 Free PMC article. - Kin28 is found within TFIIH and a Kin28-Ccl1-Tfb3 trimer complex with differential sensitivities to T-loop phosphorylation.
Keogh MC, Cho EJ, Podolny V, Buratowski S. Keogh MC, et al. Mol Cell Biol. 2002 Mar;22(5):1288-97. doi: 10.1128/MCB.22.5.1288-1297.2002. Mol Cell Biol. 2002. PMID: 11839796 Free PMC article. - Mutations in the RING domain of TFB3, a subunit of yeast transcription factor IIH, reveal a role in cell cycle progression.
Jona G, Livi LL, Gileadi O. Jona G, et al. J Biol Chem. 2002 Oct 18;277(42):39409-16. doi: 10.1074/jbc.M202733200. Epub 2002 Aug 9. J Biol Chem. 2002. PMID: 12176978 - The cdk-activating kinase (CAK): from yeast to mammals.
Kaldis P. Kaldis P. Cell Mol Life Sci. 1999 Feb;55(2):284-96. doi: 10.1007/s000180050290. Cell Mol Life Sci. 1999. PMID: 10188587 Free PMC article. Review. - The regulation and functions of cdk7.
Shuttleworth J. Shuttleworth J. Prog Cell Cycle Res. 1995;1:229-40. doi: 10.1007/978-1-4615-1809-9_18. Prog Cell Cycle Res. 1995. PMID: 9552366 Review.
Cited by
- T-loop phosphorylation stabilizes the CDK7-cyclin H-MAT1 complex in vivo and regulates its CTD kinase activity.
Larochelle S, Chen J, Knights R, Pandur J, Morcillo P, Erdjument-Bromage H, Tempst P, Suter B, Fisher RP. Larochelle S, et al. EMBO J. 2001 Jul 16;20(14):3749-59. doi: 10.1093/emboj/20.14.3749. EMBO J. 2001. PMID: 11447116 Free PMC article. - Adenosine monophosphoramidase activity of Hint and Hnt1 supports function of Kin28, Ccl1, and Tfb3.
Bieganowski P, Garrison PN, Hodawadekar SC, Faye G, Barnes LD, Brenner C. Bieganowski P, et al. J Biol Chem. 2002 Mar 29;277(13):10852-60. doi: 10.1074/jbc.M111480200. Epub 2002 Jan 22. J Biol Chem. 2002. PMID: 11805111 Free PMC article. - Specificity of Cdk activation in vivo by the two Caks Mcs6 and Csk1 in fission yeast.
Hermand D, Westerling T, Pihlak A, Thuret JY, Vallenius T, Tiainen M, Vandenhaute J, Cottarel G, Mann C, Mäkelä TP. Hermand D, et al. EMBO J. 2001 Jan 15;20(1-2):82-90. doi: 10.1093/emboj/20.1.82. EMBO J. 2001. PMID: 11226158 Free PMC article. - Inability to enter S phase and defective RNA polymerase II CTD phosphorylation in mice lacking Mat1.
Rossi DJ, Londesborough A, Korsisaari N, Pihlak A, Lehtonen E, Henkemeyer M, Mäkelä TP. Rossi DJ, et al. EMBO J. 2001 Jun 1;20(11):2844-56. doi: 10.1093/emboj/20.11.2844. EMBO J. 2001. PMID: 11387217 Free PMC article. - Structural basis of Cdk7 activation by dual T-loop phosphorylation.
Düster R, Anand K, Binder SC, Schmitz M, Gatterdam K, Fisher RP, Geyer M. Düster R, et al. bioRxiv [Preprint]. 2024 Feb 14:2024.02.14.580246. doi: 10.1101/2024.02.14.580246. bioRxiv. 2024. PMID: 38405971 Free PMC article. Updated. Preprint.
References
- Akoulitchev S, Mäkelä T P, Weinberg R A, Reinberg D. Requirement for TFIIH kinase activity in transcription by RNA polymerase II. Nature. 1995;377:557–560. - PubMed
- Albertyn J, Hohmann S, Thevelein J M, Prior B A. GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol. 1994;14:4135–4144. - PMC - PubMed
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. J. Boston, Mass: Wiley & Sons, Inc.; 1995.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous