Effect of peroxisome proliferator-activated receptor alpha activators on tumor necrosis factor expression in mice during endotoxemia - PubMed (original) (raw)
Effect of peroxisome proliferator-activated receptor alpha activators on tumor necrosis factor expression in mice during endotoxemia
M R Hill et al. Infect Immun. 1999 Jul.
Abstract
Inflammatory mediators orchestrate the host immune and metabolic response to acute bacterial infections and mediate the events leading to septic shock. Tumor necrosis factor (TNF) has long been identified as one of the proximal mediators of endotoxin action. Recent studies have implicated peroxisome proliferator-activated receptor alpha (PPARalpha) as a potential target to modulate regulation of the immune response. Since PPARalpha activators, which are hypolipidemic drugs, are being prescribed for a significant population of older patients, it is important to determine the impact of these drugs on the host response to acute inflammation. Therefore, we examined the role of PPARalpha activators on the regulation of TNF expression in a mouse model of endotoxemia. CD-1 mice treated with dietary fenofibrate or Wy-14,643 had fivefold-higher lipopolysaccharide (LPS)-induced TNF plasma levels than LPS-treated control-fed animals. Higher LPS-induced TNF levels in drug-fed animals were reflected physiologically in significantly lower glucose levels in plasma and a significantly lower 50% lethal dose than those in LPS-treated control-fed animals. Utilizing PPARalpha wild-type (WT) and knockout (KO) mice, we showed that the effect of fenofibrate on LPS-induced TNF expression was indeed mediated by PPARalpha. PPARalpha WT mice fed fenofibrate also had a fivefold increase in LPS-induced TNF levels in plasma compared to control-fed animals. However, LPS-induced TNF levels were significantly decreased and glucose levels in plasma were significantly increased in PPARalpha KO mice fed fenofibrate compared to those in control-fed animals. Data from peritoneal macrophage studies indicate that Wy-14,643 modestly decreased TNF expression in vitro. Similarly, overexpression of PPARalpha in 293T cells decreased activity of a human TNF promoter-luciferase construct. The results from these studies suggest that any anti-inflammatory activity of PPARalpha in vivo can be masked by other systemic effects of PPARalpha activators.
Figures
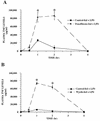
FIG. 1
Time course of fenofibrate (A) and Wy 14,643 (B) on TNF levels in plasma from mice during endotoxemia. CD-1 mice were fed rodent chow containing no drug, fenofibrate (0.5%), or Wy-14,643 (0.1%) for 2 weeks. The animals were then challenged with 0.5 ml of saline or LPS (12 mg/kg), and plasma samples were collected at the indicated times. The data are taken from a representative experiment (n = 4 animals per group), and the values are expressed as the means ± standard errors of the means. Asterisks denote values that are significantly different (P < 0.05), as determined by Student’s t test, from those of LPS-treated control-fed animals. The values for saline-treated animals were not plotted, since no TNF was detected in the plasma samples.
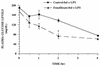
FIG. 2
Time course of fenofibrate on glucose levels in plasma from mice during endotoxemia. CD-1 mice were fed rodent chow containing no drug or fenofibrate (0.5%) for 2 weeks. The animals were then challenged with 0.5 ml of saline or LPS (12 mg/kg), and plasma samples were collected at the indicated times. The data are taken from a representative experiment (n = four animals per group), and the values are expressed as means ± standard errors of the means. Asterisks denote values that are significantly different (P < 0.05), as determined by Student’s t test, from LPS-treated control-fed animals.
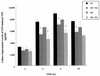
FIG. 3
Time course of Wy-14,643 on LPS-induced TNF expression in primary macrophages from PPARα wild-type (WT) versus knockout (KO) mice. Culture supernatant fractions were collected from LPS-treated (10 ng/ml) primary peritoneal macrophages at the times indicated. The data presented are from a representative experiment (n = 4 wells per group). Asterisks denote values that are significantly different (P < 0.05), as determined by Student’s t test, from LPS-treated cells in the absence of Wy-14,643.
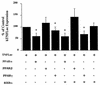
FIG. 4
Effect of overexpression of PPARs and RXRα on hTNF-luciferase activity. 293T cells were cotransfected, by using the calcium phosphate method, with a TNF promoter (−615 to +90)-luciferase construct plus PPARα, PPARβ, or PPARγ with or without RXRα. The medium was changed 24 h after transfection, and cell extracts were harvested 24 h later. Luciferase activity and protein concentration were determined, and the results are expressed as the percentage of activity of hTNF-luciferase alone. The results represent four independent experiments (n = 3 wells/group/experiment).
Similar articles
- Peroxisome proliferator-activated receptor activators inhibit lipopolysaccharide-induced tumor necrosis factor-alpha expression in neonatal rat cardiac myocytes.
Takano H, Nagai T, Asakawa M, Toyozaki T, Oka T, Komuro I, Saito T, Masuda Y. Takano H, et al. Circ Res. 2000 Sep 29;87(7):596-602. doi: 10.1161/01.res.87.7.596. Circ Res. 2000. PMID: 11009565 - Peroxisome proliferator-activated receptor alpha-independent effects of peroxisome proliferators on cysteinyl leukotriene production in mast cells.
Yamashita M. Yamashita M. Eur J Pharmacol. 2007 Feb 5;556(1-3):172-80. doi: 10.1016/j.ejphar.2006.10.032. Epub 2006 Oct 19. Eur J Pharmacol. 2007. PMID: 17113579 - PPARalpha activators inhibit tissue factor expression and activity in human monocytes.
Marx N, Mackman N, Schönbeck U, Yilmaz N, Hombach V, Libby P, Plutzky J. Marx N, et al. Circulation. 2001 Jan 16;103(2):213-9. doi: 10.1161/01.cir.103.2.213. Circulation. 2001. PMID: 11208679 - Fibrates suppress fibrinogen gene expression in rodents via activation of the peroxisome proliferator-activated receptor-alpha.
Kockx M, Gervois PP, Poulain P, Derudas B, Peters JM, Gonzalez FJ, Princen HM, Kooistra T, Staels B. Kockx M, et al. Blood. 1999 May 1;93(9):2991-8. Blood. 1999. PMID: 10216095 - Mechanism of action of the nongenotoxic peroxisome proliferators: role of the peroxisome proliferator-activator receptor alpha.
Gonzalez FJ, Peters JM, Cattley RC. Gonzalez FJ, et al. J Natl Cancer Inst. 1998 Nov 18;90(22):1702-9. doi: 10.1093/jnci/90.22.1702. J Natl Cancer Inst. 1998. PMID: 9827524 Review.
Cited by
- Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors.
Mandard S, Patsouris D. Mandard S, et al. PPAR Res. 2013;2013:613864. doi: 10.1155/2013/613864. Epub 2013 Mar 7. PPAR Res. 2013. PMID: 23577023 Free PMC article. - Downregulation of peroxisome proliferator-activated receptors (PPARs) in nasal polyposis.
Cardell LO, Hägge M, Uddman R, Adner M. Cardell LO, et al. Respir Res. 2005 Nov 7;6(1):132. doi: 10.1186/1465-9921-6-132. Respir Res. 2005. PMID: 16271155 Free PMC article. Clinical Trial. - Peroxisome Proliferator-Activated Receptors and the Hallmarks of Cancer.
Wagner N, Wagner KD. Wagner N, et al. Cells. 2022 Aug 5;11(15):2432. doi: 10.3390/cells11152432. Cells. 2022. PMID: 35954274 Free PMC article. Review. - Nuclear receptors and autoimmune disease: the potential of PPAR agonists to treat multiple sclerosis.
Racke MK, Gocke AR, Muir M, Diab A, Drew PD, Lovett-Racke AE. Racke MK, et al. J Nutr. 2006 Mar;136(3):700-3. doi: 10.1093/jn/136.3.700. J Nutr. 2006. PMID: 16484546 Free PMC article. Review. - Characterization of the adipose tissue atrophy induced by peroxisome proliferators in mice.
Xie Y, Yang Q, Nelson BD, DePierre JW. Xie Y, et al. Lipids. 2002 Feb;37(2):139-46. doi: 10.1007/s11745-002-0873-7. Lipids. 2002. PMID: 11908906
References
- Akira S, Hirano T, Taga T, Kishimoto T. Biology of multifunctional cytokines: IL-6 and related molecules (IL 1 and TNF) FASEB J. 1990;4:2860–2867. - PubMed
- Baeuerle P A, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. - PubMed
- Blok W L, Katan M B, van der Meer J. Modulation of inflammation and cytokine production by dietary (n-3) fatty acids. J Nutr. 1996;126:1515–1533. - PubMed
- Bojes H K, Germolec D R, Simeonova P, Bruccoleri A, Schoonhoven R, Luster M I, Thurman R G. Antibodies to tumor necrosis factor alpha prevent increases in cell replication in liver due to the potent peroxisome proliferator, Wy-14,643. Carcinogenesis. 1997;18:669–674. - PubMed
- Chengyu J, Ting A T, Seed B. PPAR gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials