Energy conservation by the H2:heterodisulfide oxidoreductase from Methanosarcina mazei Gö1: identification of two proton-translocating segments - PubMed (original) (raw)
Energy conservation by the H2:heterodisulfide oxidoreductase from Methanosarcina mazei Gö1: identification of two proton-translocating segments
T Ide et al. J Bacteriol. 1999 Jul.
Abstract
The membrane-bound H2:heterodisulfide oxidoreductase system of the methanogenic archaeon Methanosarcina mazei Gö1 catalyzed the H2-dependent reduction of 2-hydroxyphenazine and the dihydro-2-hydroxyphenazine-dependent reduction of the heterodisulfide of HS-CoM and HS-CoB (CoM-S-S-CoB). Washed inverted vesicles of this organism were found to couple both processes with the transfer of protons across the cytoplasmic membrane. The maximal H+/2e- ratio was 0.9 for each reaction. The electrochemical proton gradient (DeltamicroH+) thereby generated was shown to drive ATP synthesis from ADP plus Pi, exhibiting stoichiometries of 0.25 ATP synthesized per two electrons transported for both partial reactions. ATP synthesis and the generation of DeltamicroH+ were abolished by the uncoupler 3,5-di-tert-butyl-4-hydroxybenzylidenemalononitrile (SF 6847). The ATP synthase inhibitor N,N'-dicyclohexylcarbodiimide did not affect H+ translocation but led to an almost complete inhibition of ATP synthesis and decreased the electron transport rates. The latter effect was relieved by the addition of SF 6847. Thus, the energy-conserving systems showed a stringent coupling which resembles the phenomenon of respiratory control. The results indicate that two different proton-translocating segments are present in the H2:heterodisulfide oxidoreductase system; the first involves the 2-hydroxyphenazine-dependent hydrogenase, and the second involves the heterodisulfide reductase.
Figures
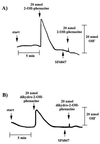
FIG. 1
Proton uptake by washed inverted vesicles from M. mazei Gö1. The experiments were performed as described in Materials and Methods. The amount of translocated protons was calculated from the difference between maximal alkalinization and the final baseline after reacidification. The reaction was started by pulses of 2-OH-phenazine or dihydro-2-OH-phenazine as indicated. SF 6847 was added as an ethanolic solution to a final concentration of 15 nmol/mg of protein. (A) H2-dependent reduction of 2-OH-phenazine under an atmosphere of molecular hydrogen; (B) dihydro-2-OH-phenazine-dependent reduction of CoM-S-S-CoB under an atmosphere of molecular nitrogen.
FIG. 2
Redox-driven ATP synthesis as catalyzed by washed inverted vesicles from M. mazei Gö1. The experiments were performed as described in Materials and Methods. The concentrations of ADP, SF 6847, and DCCD were 0.16 mM, 25 μM, and 250 nmol/mg of protein, respectively. (A) ATP synthesis in the course of H2-dependent-2-OH-phenazine reduction. The glass cuvette was gassed with H2 and contained 600 μl of buffer A, 1.6 mM AMP, and 125 μM 2-OH-phenazine. The reaction was started by the addition of washed vesicles (14 μg of protein). The following additions were made: ADP (■), ADP and SF 6847 (▵), ADP and DCCD (▴), ADP, SF 6847, and DCCD (●), ADP under N2 (○). □, ADP omitted. (B) ATP synthesis coupled to dihydro-2-OH-phenazine-dependent heterodisulfide reduction. Details of the experiment and symbols are as described for panel A except that (i) the reaction mixture contained dihydro-2-OH-phenazine (instead of 2-OH-phenazine) and 240 nmol of CoM-S-S-CoB and (ii) open circles represent assays with ADP added and CoM-S-S-CoB omitted. The atmosphere was N2.
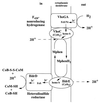
FIG. 3
Tentative scheme of membrane-bound electron transfer coupled to proton translocation in M. mazei Gö1. Mphen, methanophenazine; MphenH2, dihydromethanophenazine; VhoG, 40-kDa subunit of the F420-nonreducing hydrogenase; VhoA, 60-kDa subunit of the F420-nonreducing hydrogenase; VhoC, cytochrome _b_1 (Cytb1) encoded by the third gene (vhoC) of the hydrogenase operon; HdrDE, subunits of the heterodisulfide reductase; FeS, iron-sulfur clusters; Ni, nickel-iron center of the F420-nonreducing hydrogenase.
Similar articles
- Inhibition of membrane-bound electron transport of the methanogenic archaeon Methanosarcina mazei Gö1 by diphenyleneiodonium.
Brodersen J, Bäumer S, Abken HJ, Gottschalk G, Deppenmeier U. Brodersen J, et al. Eur J Biochem. 1999 Jan;259(1-2):218-24. doi: 10.1046/j.1432-1327.1999.00017.x. Eur J Biochem. 1999. PMID: 9914496 - The F420H2:heterodisulfide oxidoreductase system from Methanosarcina species. 2-Hydroxyphenazine mediates electron transfer from F420H2 dehydrogenase to heterodisulfide reductase.
Bäumer S, Murakami E, Brodersen J, Gottschalk G, Ragsdale SW, Deppenmeier U. Bäumer S, et al. FEBS Lett. 1998 May 29;428(3):295-8. doi: 10.1016/s0014-5793(98)00555-9. FEBS Lett. 1998. PMID: 9654152 - The F420H2 dehydrogenase from Methanosarcina mazei is a Redox-driven proton pump closely related to NADH dehydrogenases.
Baumer S, Ide T, Jacobi C, Johann A, Gottschalk G, Deppenmeier U. Baumer S, et al. J Biol Chem. 2000 Jun 16;275(24):17968-73. doi: 10.1074/jbc.M000650200. J Biol Chem. 2000. PMID: 10751389 - Novel reactions involved in energy conservation by methanogenic archaea.
Deppenmeier U, Lienard T, Gottschalk G. Deppenmeier U, et al. FEBS Lett. 1999 Sep 3;457(3):291-7. doi: 10.1016/s0014-5793(99)01026-1. FEBS Lett. 1999. PMID: 10471795 Review. - The membrane-bound electron transport system of Methanosarcina species.
Deppenmeier U. Deppenmeier U. J Bioenerg Biomembr. 2004 Feb;36(1):55-64. doi: 10.1023/b:jobb.0000019598.64642.97. J Bioenerg Biomembr. 2004. PMID: 15168610 Review.
Cited by
- DNA microarray analysis of Methanosarcina mazei Gö1 reveals adaptation to different methanogenic substrates.
Hovey R, Lentes S, Ehrenreich A, Salmon K, Saba K, Gottschalk G, Gunsalus RP, Deppenmeier U. Hovey R, et al. Mol Genet Genomics. 2005 May;273(3):225-39. doi: 10.1007/s00438-005-1126-9. Epub 2005 Apr 7. Mol Genet Genomics. 2005. PMID: 15902489 - Transcriptional response to prolonged perchlorate exposure in the methanogen Methanosarcina barkeri and implications for Martian habitability.
Harris RL, Schuerger AC, Wang W, Tamama Y, Garvin ZK, Onstott TC. Harris RL, et al. Sci Rep. 2021 Jun 11;11(1):12336. doi: 10.1038/s41598-021-91882-0. Sci Rep. 2021. PMID: 34117335 Free PMC article. - Genomic analysis of methanogenic archaea reveals a shift towards energy conservation.
Gilmore SP, Henske JK, Sexton JA, Solomon KV, Seppälä S, Yoo JI, Huyett LM, Pressman A, Cogan JZ, Kivenson V, Peng X, Tan Y, Valentine DL, O'Malley MA. Gilmore SP, et al. BMC Genomics. 2017 Aug 21;18(1):639. doi: 10.1186/s12864-017-4036-4. BMC Genomics. 2017. PMID: 28826405 Free PMC article. - H2 Kinetic Isotope Fractionation Superimposed by Equilibrium Isotope Fractionation During Hydrogenase Activity of D. vulgaris Strain Miyazaki.
Löffler M, Kümmel S, Vogt C, Richnow HH. Löffler M, et al. Front Microbiol. 2019 Jul 10;10:1545. doi: 10.3389/fmicb.2019.01545. eCollection 2019. Front Microbiol. 2019. PMID: 31354654 Free PMC article. - Function of Ech hydrogenase in ferredoxin-dependent, membrane-bound electron transport in Methanosarcina mazei.
Welte C, Kallnik V, Grapp M, Bender G, Ragsdale S, Deppenmeier U. Welte C, et al. J Bacteriol. 2010 Feb;192(3):674-8. doi: 10.1128/JB.01307-09. Epub 2009 Nov 30. J Bacteriol. 2010. PMID: 19948802 Free PMC article.
References
- Bäumer S, Murakami E, Brodersen J, Gottschalk G, Ragsdale S W, Deppenmeier U. The F420H2:heterodisulfide oxidoreductase system from Methanosarcina species. FEBS Lett. 1998;428:295–298. - PubMed
- Blaut M, Müller V, Gottschalk G. Proton translocation coupled to methanogenesis from methanol+hydrogen in Methanosarcina barkeri. FEBS Lett. 1987;215:53–57.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous