G-protein-coupled receptor heterodimerization modulates receptor function - PubMed (original) (raw)
. 1999 Jun 17;399(6737):697-700.
doi: 10.1038/21441.
Affiliations
- PMID: 10385123
- PMCID: PMC3125690
- DOI: 10.1038/21441
G-protein-coupled receptor heterodimerization modulates receptor function
B A Jordan et al. Nature. 1999.
Abstract
The opioid system modulates several physiological processes, including analgesia, the stress response, the immune response and neuroendocrine function. Pharmacological and molecular cloning studies have identified three opioid-receptor types, delta, kappa and mu, that mediate these diverse effects. Little is known about the ability of the receptors to interact to form new functional structures, the simplest of which would be a dimer. Structural and biochemical studies show that other G-protein-coupled receptors (GPCRs) interact to form homodimers. Moreover, two non-functional receptors heterodimerize to form a functional receptor, suggesting that dimerization is crucial for receptor function. However, heterodimerization between two fully functional receptors has not been documented. Here we provide biochemical and pharmacological evidence for the heterodimerization of two fully functional opioid receptors, kappa and delta. This results in a new receptor that exhibits ligand binding and functional properties that are distinct from those of either receptor. Furthermore, the kappa-delta heterodimer synergistically binds highly selective agonists and potentiates signal transduction. Thus, heterodimerization of these GPCRs represents a novel mechanism that modulates their function.
Figures
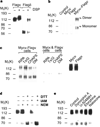
Figure 1
Characteristics of κ-opioid-receptor homodimers. a, Immunoblotting of lysates from cells expressing Flag–κ receptors or Flag–δ receptors. b, c, _Myc_-tagged κ-receptors can be co-precipitated only from cells expressing both myc and Flag-tagged receptors (b) under a variety of extraction conditions and not from a mixture of cells individually expressing these receptors (c). Expression of _myc_- or Flag-tagged receptors was confirmed by immunoblotting with the appropriate antisera (right panel). d, e, Treatment of cells expressing κ-receptors with 1 mM DTT for 30 min followed by 5 mM iodacetamide (IAM) or _N_-ethylmaleimide (NEM) results in monomerization (d), whereas treatment with 100 nM agonists for 60 min does not (e). Immunoblotting experiments used anti-Flag antibodies; immunoprecipitation experiments used anti-myc antibodies.
Figure 2
Characterization of κ–δ heterodimers. a, κ–δ heterodimers can be immunoprecipitated only from _myc_–κ- and Flag-δ-expressing cells and not from _myc_–κ- and Flag–µ-expressing cells. b, κ–δ heterodimers can be immunoprecipitated under a variety of extraction conditions and not from a mixture of cells individually expressing these receptors. c, Expression of _myc_- or Flag-tagged receptors in each cell line was confirmed by immunoblotting with the appropriate antisera (right panel). Treatment with β-mercaptoethanol 5% (β–ME) for 5 min results in the destabilization of dimers. d, Internalization of receptors in response to 1 µM etorphine for 60 min. Stippled bars, _myc_–δ; shaded bars, Flag–κ. Significant differences from untreated controls are indicated; *P < 0.05; ***P < 0.005 (n = 3). Immunoblotting experiments used anti-Flag antibodies; immunoprecipitation experiments used anti-myc antibodies.
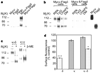
Figure 3
Ligand binding and functional properties. a–c, Competition of 3H-diprenorphine binding by U69593 (square), norbinaltorphimine (triangle), diprenorphine (star), DPDPE (circle) and TIPPΨ (diamond) in membranes from cells expressing κ- (a), δ- (b) or κ- and δ- (c) receptors. d, Displacement of 3H-diprenorphine by U69593 in the presence of 10 µM DPDPE (triangle) or DPDPE in the presence of 10 µM U69593 (diamond). e, f, Decrease in intracellular cAMP (e) or increase in phospho-MAPK (f) by U69593 (square), DPDPE (circle) or U69593 + DPDPE (triangle). In e, the 50% inhibitory concentrations (nM) were: U69593, 1.3 ± 0.7; DPDPE, 0.9 ± 0.4; U69593 + DPDPE, 0.06 ± 0.03. Activation of homodimers in these cells could account for the effect seen by individual agonists. Error bars represent s.e.m. (n = 3–4).
Similar articles
- Differential coupling of mu-, delta-, and kappa-opioid receptors to G alpha16-mediated stimulation of phospholipase C.
Lee JW, Joshi S, Chan JS, Wong YH. Lee JW, et al. J Neurochem. 1998 May;70(5):2203-11. J Neurochem. 1998. PMID: 9572309 - Opioid modulation of extracellular signal-regulated protein kinase activity is ras-dependent and involves Gbetagamma subunits.
Belcheva MM, Vogel Z, Ignatova E, Avidor-Reiss T, Zippel R, Levy R, Young EC, Barg J, Coscia CJ. Belcheva MM, et al. J Neurochem. 1998 Feb;70(2):635-45. doi: 10.1046/j.1471-4159.1998.70020635.x. J Neurochem. 1998. PMID: 9453557 Free PMC article. - Selective interference of beta-arrestin 1 with kappa and delta but not mu opioid receptor/G protein coupling.
Cheng ZJ, Yu QM, Wu YL, Ma L, Pei G. Cheng ZJ, et al. J Biol Chem. 1998 Sep 18;273(38):24328-33. doi: 10.1074/jbc.273.38.24328. J Biol Chem. 1998. PMID: 9733719 - Opioid receptor types and subtypes: the delta receptor as a model.
Zaki PA, Bilsky EJ, Vanderah TW, Lai J, Evans CJ, Porreca F. Zaki PA, et al. Annu Rev Pharmacol Toxicol. 1996;36:379-401. doi: 10.1146/annurev.pa.36.040196.002115. Annu Rev Pharmacol Toxicol. 1996. PMID: 8725395 Review. - Functional analysis of cloned opioid receptors in transfected cell lines.
Piros ET, Hales TG, Evans CJ. Piros ET, et al. Neurochem Res. 1996 Nov;21(11):1277-85. doi: 10.1007/BF02532368. Neurochem Res. 1996. PMID: 8947917 Review.
Cited by
- Neuropathic Pain due to Small Fiber Neuropathy in Aging: Current Management and Future Prospects.
Brouwer BA, de Greef BT, Hoeijmakers JG, Geerts M, van Kleef M, Merkies IS, Faber CG. Brouwer BA, et al. Drugs Aging. 2015 Aug;32(8):611-21. doi: 10.1007/s40266-015-0283-8. Drugs Aging. 2015. PMID: 26239827 Free PMC article. Review. - Desensitization, trafficking, and resensitization of the pituitary thyrotropin-releasing hormone receptor.
Hinkle PM, Gehret AU, Jones BW. Hinkle PM, et al. Front Neurosci. 2012 Dec 13;6:180. doi: 10.3389/fnins.2012.00180. eCollection 2012. Front Neurosci. 2012. PMID: 23248581 Free PMC article. - Effects of mu- and kappa-2 opioid receptor agonists on pain and rearing behaviors.
Neubert JK, Rossi HL, Pogar J, Jenkins AC, Caudle RM. Neubert JK, et al. Behav Brain Funct. 2007 Sep 20;3:49. doi: 10.1186/1744-9081-3-49. Behav Brain Funct. 2007. PMID: 17883847 Free PMC article. - A gene-based method for detecting gene-gene co-association in a case-control association study.
Peng Q, Zhao J, Xue F. Peng Q, et al. Eur J Hum Genet. 2010 May;18(5):582-7. doi: 10.1038/ejhg.2009.223. Epub 2009 Dec 23. Eur J Hum Genet. 2010. PMID: 20029457 Free PMC article. - Each rhodopsin molecule binds its own arrestin.
Hanson SM, Gurevich EV, Vishnivetskiy SA, Ahmed MR, Song X, Gurevich VV. Hanson SM, et al. Proc Natl Acad Sci U S A. 2007 Feb 27;104(9):3125-8. doi: 10.1073/pnas.0610886104. Epub 2007 Feb 20. Proc Natl Acad Sci U S A. 2007. PMID: 17360618 Free PMC article.
References
- Herz A. Opioids. Vol. 1. Berlin: Springer; 1993.
- Miotto K, Magendzo K, Evans CJ. In: The Pharmacology of Opioid Peptides. Tseng L, editor. Harwood, Singapore: 1995. pp. 57–71.
- Hebert TE, Bouvier M. Structural and functional aspects of G protein-coupled receptor oligomerization. Biochem. Cell Biol. 1998;76:1–11. - PubMed
- Gouldson PR, Snell CR, Bywater RP, Higgs C, Reynolds CA. Domain swapping in G-protein coupled receptor dimers. Protein Eng. 1998;11:1181–1193. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials