Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53 - PubMed (original) (raw)
Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53
K A Webster et al. J Clin Invest. 1999 Aug.
Abstract
Ischemia and reperfusion activate cardiac myocyte apoptosis, which may be an important feature in the progression of ischemic heart disease. The relative contributions of ischemia and reperfusion to apoptotic signal transduction have not been established. We report here that severe chronic hypoxia alone does not cause apoptosis of cardiac myocytes in culture. When rapidly contracting cardiac myocytes were exposed to chronic hypoxia, apoptosis occurred only when there was a decrease in extracellular pH ([pH](o)). Apoptosis did not occur when [pH](o) was neutralized. Addition of acidic medium from hypoxic cultures or exogenous lactic acid stimulated apoptosis in aerobic myocytes. Hypoxia-acidosis-mediated cell death was independent of p53: equivalent apoptosis occurred in cardiac myocytes isolated from wild-type and p53 knockout mice, and hypoxia caused no detectable change in p53 abundance or p53-dependent transcription. Reoxygenation of hypoxic cardiac myocytes induced apoptosis in 25-30% of the cells and was also independent of p53 by the same criteria. Finally, equivalent levels of apoptosis, as demonstrated by DNA fragmentation, were induced by ischemia-reperfusion, but not by ischemia alone, of Langendorff-perfused hearts from wild-type and p53 knockout mice. We conclude that acidosis, reoxygenation, and reperfusion, but not hypoxia (or ischemia) alone, are strong stimuli for programmed cell death that is substantially independent of p53.
Figures
Figure 1
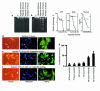
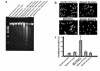
Contributions of waste metabolic buildup to apoptosis induced by chronic hypoxia. (a and b) Parallel cultures of cardiac myocytes were exposed to hypoxia as described in Methods. In a, the medium was replaced with fresh hypoxic medium every 12 hours; in b, there was no medium replacement. Cultures were harvested at the indicated times and processed for DNA fragmentation. (c) Intracellular ATP, medium glucose, and [pH]o were measured in parallel cultures as described in Methods; results are means of 3 separate experiments. Open circles are results from cultures without medium replacement; closed circles, with medium replacement. (d–f) Typical fields of myocytes stained with
HOECHST
33342 and anti-myosin antibody as described in Methods. (g) Quantitations of
HOECHST
-stained condensed nuclei, also described in Methods. At 24 and 48 hours, less than 2% of cells were PI positive (scored as necrotic) under any condition; at 72 hours, hypoxia-acidic cultures had more PI-positive cells, and these were scored as apoptotic if they contained condensed nuclei. Costaining populations were not distinguished from PI-excluding cells at this stage. Results are representative of at least 3 experiments.
Figure 2
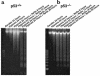
Induction of apoptosis by conditioned medium. Cultures were grown under hypoxia with or without medium change. After 48 hours, the medium was removed and the cells were analyzed for DNA fragmentation (top). The spent medium was centrifuged at 800 g for 5 minutes, to pellet cells and debris, and was added directly to a second set of plates. These plates were incubated in air or under hypoxia as indicated. After 24 hours, these cells were also harvested and analyzed for DNA fragmentation. Controls show untreated cells harvested at the time of medium change. [pH]o was measured in all cases immediately before harvesting the cells. Control samples shown in the last 2 lanes of the bottom left panel did not receive spent medium. Results are representative of 3 separate experiments.
Figure 3
Neutralized medium prevents apoptosis. Conditioned medium was generated as described in Figure 2. (a, top) DNA ladders from the cardiac myocytes used to generate the spent medium. (a, bottom) Spent medium was added directly to fresh plates of cardiac myocytes (middle 2 lanes), or it was neutralized to pH 7.4 with HEPES (20 mM final concentration) and NaOH and then added to a second set of fresh cardiac myocytes. Both sets of plates were incubated under hypoxia for 24 hours and analyzed for DNA fragmentation. Control plates were incubated under aerobic or hypoxic conditions in parallel. (b) Parallel sets of cardiac myocytes exposed to hypoxia without medium change. In the first set (b, left), the acid was allowed to accumulate exactly as described in Figure 1b; in the second set (b, right) aliquots of 250 mM HEPES and 250 mM NaOH were added every 12 hours to maintain a [pH]o of approximately 7.1. Measurements of DNA fragmentation and determinations of percent condensed nuclei (c) were as described in Methods. In all cases, the medium pH was measured immediately before the cultures were harvested. (d) Typical field of myocytes stained with
HOECHST
33342 and anti-myosin antibody as described in Figure 1d. Results in a, b, and c are from typical experiments; error bars in c are SEM (n = 3).
Figure 4
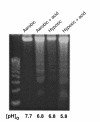
Exogenous lactic acid induces apoptosis. Lactic acid was added to culture medium to a final concentration of 16.5 mM, and the pH was adjusted to 6.8 by adding phosphoric acid. The medium was added to fresh cultures of cardiac myocytes, and the cultures were incubated under air or hypoxia as indicated. DNA fragmentation was determined as described in Methods. The initial pH in both aerobic and hypoxic cultures was 6.8; the aerobic cultures maintained this pH, but the pH of hypoxic cultures decreased further to a final pH of 5.8.
Figure 5
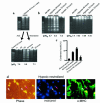
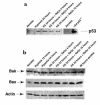
Hypoxia-acidosis–mediated apoptosis is independent of p53. (a) Cardiac myocytes were exposed to hypoxia as described in Methods. Plates were harvested at the indicated times, and proteins were extracted. For the 36-hour time points in a, the culture medium was changed every 12 hours (no metabolite buildup) or there was no medium change (metabolite buildup). In the bottom panel, proteins were extracted from MEFs as described in Methods. Western blots and probes are described in Methods. (b) Nuclear extracts were prepared from aerobic and (unfed) hypoxic cultures and from cultures infected for 48 hours with an adenoviral vector expressing p53 as described in Methods. Competition lanes include self (p53) and an oligonucleotide with the hypoxia-inducible factor-1 consensus binding site (HRE) (73). All probes and procedures are described in Methods. Equal amounts of protein (8 μg) were loaded in each lane. (c) Cardiac myocytes were exposed to hypoxia (without medium change) for the indicated times; protein was extracted and analyzed by Western blot using anti-Bax, anti-Bak, and anti-actin antibodies, described in Methods. (d) Cardiac myocytes were transfected with the indicated plasmid using calcium phosphate as described in Methods. Transfected cultures were incubated under aerobic or hypoxic conditions for 24, 48, or 72 hours (without medium change) as indicated. For viral infections, transfected cultures were inoculated with wild-type p53 at 5 PFU/cell plus 5 PFU of a virus expressing LacZ (Ad-p53), or with 5 PFU/cell of wild-type p53 plus 5 PFU of dominant-negative p53 (Ad-p53DN; contains a Cys-135→Ser mutation; ref. 74) as described in Methods. (e) Cardiac myocytes were isolated from wild-type or p53 knockout neonatal mice as described in Methods. The myocytes were cultured and exposed to hypoxia under the same conditions described in Figure 1b.
Figure 6
Induction of apoptosis by hypoxia-reoxygenation. Cultures of cardiac myocytes were exposed to hypoxia for 24 hours and then reoxygenated by replacing the medium with fresh aerobic medium and incubating in air (21% O2/5% CO2). Plates were harvested at the indicated times and analyzed for DNA fragmentation (a) or nuclear condensation (b and c) as described in Methods.
HOECHST
staining and the quantitation of condensed nuclei were as described in Figure 1. Typical fields of
HOECHST
-stained cardiac myocytes (b) were photographed at ×400. The 48-hour hypoxic incubation (c) was subjected to medium replacement and did not become acidic.
Figure 7
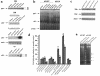
Hypoxia and reoxygenation do not affect expression of p53, Bak, or Bax. Cultures of cardiac myocytes were exposed to hypoxia for 24 hours and then to reoxygenation as described in Figure 6. Proteins were extracted and analyzed by Western blots as described in Methods.
Figure 8
Induction of apoptosis in cardiac myocytes from p53–/– knockout mice. Cardiac myocytes were isolated from 1- to 2-day-old wild-type (a) or p53-nullizygous (b) mice as described in Methods. Cell culture and treatments were exactly as described in Figure 6.
Figure 9
Nucleosomal fragmentation in genomic DNA from ischemia-reperfused mouse hearts. Hearts were perfused on a Langendorff apparatus as described in Methods. Left ventricle DNA was isolated from wild-type and p53–/– knockout mice subjected to ischemia alone or to ischemia and reperfusion. Controls were perfused with aerobic perfusion buffer (95% O2/5% CO2) for 3 hours. Nucleosomal ladders are apparent from the ischemia-reperfused hearts of both genotypes.
Similar articles
- p53 and the hypoxia-induced apoptosis of cultured neonatal rat cardiac myocytes.
Long X, Boluyt MO, Hipolito ML, Lundberg MS, Zheng JS, O'Neill L, Cirielli C, Lakatta EG, Crow MT. Long X, et al. J Clin Invest. 1997 Jun 1;99(11):2635-43. doi: 10.1172/JCI119452. J Clin Invest. 1997. PMID: 9169493 Free PMC article. - A cardiac myocyte-restricted Lin28/let-7 regulatory axis promotes hypoxia-mediated apoptosis by inducing the AKT signaling suppressor PIK3IP1.
Joshi S, Wei J, Bishopric NH. Joshi S, et al. Biochim Biophys Acta. 2016 Feb;1862(2):240-51. doi: 10.1016/j.bbadis.2015.12.004. Epub 2015 Dec 2. Biochim Biophys Acta. 2016. PMID: 26655604 Free PMC article. - The pH paradox in ischemia-reperfusion injury to cardiac myocytes.
Lemasters JJ, Bond JM, Chacon E, Harper IS, Kaplan SH, Ohata H, Trollinger DR, Herman B, Cascio WE. Lemasters JJ, et al. EXS. 1996;76:99-114. doi: 10.1007/978-3-0348-8988-9_7. EXS. 1996. PMID: 8805791 Review. - Targeting MIAT reduces apoptosis of cardiomyocytes after ischemia/reperfusion injury.
Chen L, Zhang D, Yu L, Dong H. Chen L, et al. Bioengineered. 2019 Dec;10(1):121-132. doi: 10.1080/21655979.2019.1605812. Bioengineered. 2019. PMID: 30971184 Free PMC article. - A unique pathway of cardiac myocyte death caused by hypoxia-acidosis.
Graham RM, Frazier DP, Thompson JW, Haliko S, Li H, Wasserlauf BJ, Spiga MG, Bishopric NH, Webster KA. Graham RM, et al. J Exp Biol. 2004 Aug;207(Pt 18):3189-200. doi: 10.1242/jeb.01109. J Exp Biol. 2004. PMID: 15299040 Review.
Cited by
- Assessment of human bioengineered cardiac tissue function in hypoxic and re-oxygenized environments to understand functional recovery in heart failure.
Yamasaki Y, Matsuura K, Sasaki D, Shimizu T. Yamasaki Y, et al. Regen Ther. 2021 Apr 10;18:66-75. doi: 10.1016/j.reth.2021.03.007. eCollection 2021 Dec. Regen Ther. 2021. PMID: 33869689 Free PMC article. - Glucose reintroduction triggers the activation of Nrf2 during experimental ischemia reperfusion.
Crean D, Felice L, Taylor CT, Rabb H, Jennings P, Leonard MO. Crean D, et al. Mol Cell Biochem. 2012 Jul;366(1-2):231-8. doi: 10.1007/s11010-012-1300-4. Epub 2012 Mar 30. Mol Cell Biochem. 2012. PMID: 22460831 - Acidic preconditioning protects endothelial cells against apoptosis through p38- and Akt-dependent Bcl-xL overexpression.
Flacke JP, Kumar S, Kostin S, Reusch HP, Ladilov Y. Flacke JP, et al. Apoptosis. 2009 Jan;14(1):90-6. doi: 10.1007/s10495-008-0287-5. Apoptosis. 2009. PMID: 19082728 Free PMC article. - Saffron (Crocus sativus) pretreatment confers cardioprotection against ischemia-reperfusion injuries in isolated rabbit heart.
Nader M, Chahine N, Salem C, Chahine R. Nader M, et al. J Physiol Biochem. 2016 Dec;72(4):711-719. doi: 10.1007/s13105-016-0510-8. Epub 2016 Aug 10. J Physiol Biochem. 2016. PMID: 27507116 - The antiarrhythmic peptide rotigaptide (ZP123) increases gap junction intercellular communication in cardiac myocytes and HeLa cells expressing connexin 43.
Clarke TC, Thomas D, Petersen JS, Evans WH, Martin PE. Clarke TC, et al. Br J Pharmacol. 2006 Mar;147(5):486-95. doi: 10.1038/sj.bjp.0706631. Br J Pharmacol. 2006. PMID: 16415913 Free PMC article.
References
- Haunstetter A, Izumo S. Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res. 1998;82:1111–1129. - PubMed
- MacLellan WR, Schneider MD. Death by design: programmed cell death in cardiovascular biology and disease. Circ Res. 1997;81:137–144. - PubMed
- Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. - PubMed
- Olivetti G, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. - PubMed
- Olivetti G, et al. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1994;28:2005–2016. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous