p21(WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential - PubMed (original) (raw)
p21(WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem-cell potential
G I Topley et al. Proc Natl Acad Sci U S A. 1999.
Abstract
p21(WAF1/Cip1) is one of the best characterized downstream targets of p53, and the growth suppressing function of this cyclin-dependent kinase inhibitor is well established. However, whether p21 exerts a tumor-suppressing function of its own remains to be established. We report here that, similarly to loss of p53, disruption of the p21(WAF1/Cip1) gene results in a markedly increased susceptibility to chemically induced skin carcinoma formation, whereas the number of papillomas is reduced. Previous evidence indicates that malignant versus benign keratinocyte tumor formation is likely to involve distinct target-cell populations with a different commitment to differentiation. In parallel with the increased susceptibility to carcinoma formation, loss of p21(WAF1/Cip1) was found to promote keratinocyte subpopulations with increased growth/differentiation potential, including clonal growth capability, reversible commitment to differentiation, and capability to generate all types of terminally differentiated keratinocytes present in vivo, not only in the interfollicular epidermis but also in hair follicles. Thus, these findings have revealed a function of p21 as a suppressor of malignant but not benign skin-tumor formation and a determinant of the growth/differentiation potential of keratinocyte subpopulations.
Figures
Figure 1
Papilloma and carcinoma formation in p21−/− versus p21+/− and p21+/+ littermates. (A) The three groups of mice were subjected to a classical chemical carcinogenesis protocol as described in Materials and Methods. The arising papillomas and carcinomas were counted regularly, and the results of macroscopic analysis were confirmed histologically when the animals were sacrificed. Each group consisted of 15–20 animals. Statistical significance was determined as indicated in the text. (B) Carcinoma formation of p21−/− versus p21+/− and p21+/+ littermates in a second independent experiment performed similarly to the first. (C) Percentage of mice with multiple independent carcinomas, as counted in the two experiments at 30 weeks after the start of tumor promotion.
Figure 2
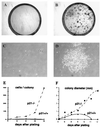
Clonogenic growth potential of p21-deficient versus wild-type keratinocytes. Primary keratinocytes derived from p21−/− and p21+/+ mice were plated under sparse conditions (105 cells per 60-mm dish) and cultivated in growth medium at low calcium concentrations (0.05 mM) for up to 2 weeks. (A and B) Culture dishes fixed and stained with 0.1% crystal violet at the end of the experiment. (C and D) Photographs of representative colonies at 10 days after plating. (E and F) Average colony size and colony cell number as a function of time, as determined by examination of several random colonies at daily intervals. Cells were tested in triplicate dishes, and similar results were observed in another independent experiment.
Figure 3
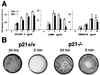
Cell adhesive properties of p21-deficient versus wild-type keratinocytes. (A) Primary keratinocytes were plated onto 96-well dishes coated with increasing amounts (0–10 μg/ml) of collagen IV, laminin, and fibronectin. After 90 min, adherent cells were fixed and stained with 0.1% crystal violet. Staining intensity was determined with an ELISA reader at 570 nm. Each point represents the average of triplicate wells, and similar results were obtained in a second independent experiment. (B) Primary keratinocytes from p21−/− and p21+/+ mice were plated on triplicate dishes in relatively large numbers (106 per 60-mm dish) and allowed to attach for either 24 h or 5 min. After 2 weeks of cultivation, dishes were fixed and stained with 0.1% crystal violet. Similar results were obtained in a second independent experiment.
Figure 4
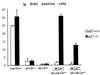
Reversible versus irreversible commitment to differentiation of primary keratinocytes in culture. BrdUrd labeling index of p21−/− versus p21+/+ keratinocytes under growing conditions in low-calcium medium, after induction of differentiation by calcium (2 mM) for the indicated number of days (d) and after induction of differentiation by calcium and subsequent incubation in low-calcium medium for an additional 2 days. The percentage of BrdUrd-positive nuclei was determined as described in Materials and Methods. In each case, four independent fields were counted (a minimum of 100 cells per field), and the SD among values from the various fields was calculated. Similar results were obtained in two independent experiments.
Figure 5
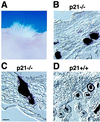
Pluripotent populations of p21−/− keratinocytes as assessed in vivo by a skin/hair-reconstitution assay. Primary keratinocytes derived from p21−/− and p21+/+ mice were infected with an AP-transducing retrovirus under conditions that resulted in 10–12.5% of cells expressing the AP marker. Cells were grafted onto nude mice together with a hair-inducing dermal cell preparation, as described in Materials and Methods. (A) Macroscopical view of hair formation on the back of a nude mouse transplanted with p21-deficient keratinocytes. Similar hair formation was observed after transplantation of wild-type cells (data not shown). (B and D) Cross section of reconstituted follicles formed by p21-knockout keratinocytes (B) and their wild-type counterparts (D). Note the uniformly labeled follicles formed by AP-positive p21−/− cells and the restricted labeling of follicles formed by AP-positive p21+/+ cells. (C) A longitudinal section of a hair follicle formed by p21-deficient keratinocytes, with uniform AP staining, extending to the attached sebaceous gland. Two independent grafting experiments were performed. Results were quantified by counting only follicles in cross section, where the various concentric regions can be distinctly visualized. Of the ≈200 AP-positive follicles that were counted in grafts of p21−/− keratinocytes, 80% showed restricted labeling to either the outer or inner root sheath and/or shaft, whereas 20% showed uniform staining of all three regions. In contrast, <1% of uniformly stained follicles were found in the p21+/+ keratinocyte grafts. [Bar = 230 μm (B and D) and 250 μm (C).]
Similar articles
- Loss of p21CIP1/WAF1 does not recapitulate accelerated malignant conversion caused by p53 loss in experimental skin carcinogenesis.
Weinberg WC, Montano NE, Deng C. Weinberg WC, et al. Oncogene. 1997 Aug 7;15(6):685-90. doi: 10.1038/sj.onc.1201230. Oncogene. 1997. PMID: 9264409 - Involvement of the cell-cycle inhibitor Cip1/WAF1 and the E1A-associated p300 protein in terminal differentiation.
Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingston DM, Dotto GP. Missero C, et al. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5451-5. doi: 10.1073/pnas.92.12.5451. Proc Natl Acad Sci U S A. 1995. PMID: 7777529 Free PMC article. - The growth-regulatory role of p21 (WAF1/CIP1).
Gartel AL, Tyner AL. Gartel AL, et al. Prog Mol Subcell Biol. 1998;20:43-71. doi: 10.1007/978-3-642-72149-6_4. Prog Mol Subcell Biol. 1998. PMID: 9928526 Review. No abstract available. - Size does matter: will knockout of p21(WAF1/CIP1) save the kidney by limiting compensatory renal growth?
Al-Awqati Q, Preisig PA. Al-Awqati Q, et al. Proc Natl Acad Sci U S A. 1999 Sep 14;96(19):10551-3. doi: 10.1073/pnas.96.19.10551. Proc Natl Acad Sci U S A. 1999. PMID: 10485857 Free PMC article. Review. No abstract available.
Cited by
- Runx1 and p21 synergistically limit the extent of hair follicle stem cell quiescence in vivo.
Lee J, Hoi CS, Lilja KC, White BS, Lee SE, Shalloway D, Tumbar T. Lee J, et al. Proc Natl Acad Sci U S A. 2013 Mar 19;110(12):4634-9. doi: 10.1073/pnas.1213015110. Epub 2013 Mar 4. Proc Natl Acad Sci U S A. 2013. PMID: 23487742 Free PMC article. - Multifaceted p21 in carcinogenesis, stemness of tumor and tumor therapy.
Xiao BD, Zhao YJ, Jia XY, Wu J, Wang YG, Huang F. Xiao BD, et al. World J Stem Cells. 2020 Jun 26;12(6):481-487. doi: 10.4252/wjsc.v12.i6.481. World J Stem Cells. 2020. PMID: 32742565 Free PMC article. Review. - Regenerative response in ischemic brain restricted by p21cip1/waf1.
Qiu J, Takagi Y, Harada J, Rodrigues N, Moskowitz MA, Scadden DT, Cheng T. Qiu J, et al. J Exp Med. 2004 Apr 5;199(7):937-45. doi: 10.1084/jem.20031385. J Exp Med. 2004. PMID: 15067031 Free PMC article. - SOCS1 Deficiency Promotes Hepatocellular Carcinoma via SOCS3-Dependent CDKN1A Induction and NRF2 Activation.
Khan MGM, Boufaied N, Yeganeh M, Kandhi R, Petkiewicz S, Sharma A, Yoshimura A, Ferbeyre G, Labbé DP, Ramanathan S, Ilangumaran S. Khan MGM, et al. Cancers (Basel). 2023 Jan 31;15(3):905. doi: 10.3390/cancers15030905. Cancers (Basel). 2023. PMID: 36765862 Free PMC article. - Loss of p21(WAF1) compartmentalisation in sebaceous carcinoma compared with sebaceous hyperplasia and sebaceous adenoma.
McBride SR, Leonard N, Reynolds NJ. McBride SR, et al. J Clin Pathol. 2002 Oct;55(10):763-6. doi: 10.1136/jcp.55.10.763. J Clin Pathol. 2002. PMID: 12354803 Free PMC article.
References
- Dover R, Wright N. In: Physiology, Biochemistry, and Molecular Biology of the Skin. Goldsmith L, editor. New York: Oxford Univ. Press; 1991. pp. 239–265.
- Cotsarelis G, Sun T T, Lavker R M. Cell. 1990;61:1329–1337. - PubMed
- Jones P H, Watt F M. Cell. 1993;73:713–724. - PubMed
- Jones P H, Harper S, Watt F M. Cell. 1995;80:83–93. - PubMed
- Moll I. J Invest Dermatol. 1995;105:14–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR 39190/AR/NIAMS NIH HHS/United States
- CA 16038/CA/NCI NIH HHS/United States
- R01 CA073796/CA/NCI NIH HHS/United States
- R01 AR039190/AR/NIAMS NIH HHS/United States
- ES 07784/ES/NIEHS NIH HHS/United States
- P01 CA016038/CA/NCI NIH HHS/United States
- P30 ES007784/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous