Characterization of a fourth adaptor-related protein complex - PubMed (original) (raw)
Characterization of a fourth adaptor-related protein complex
J Hirst et al. Mol Biol Cell. 1999 Aug.
Free PMC article
Abstract
Adaptor protein complexes (APs) function as vesicle coat components in different membrane traffic pathways; however, there are a number of pathways for which there is still no candidate coat. To find novel coat components related to AP complexes, we have searched the expressed sequence tag database and have identified, cloned, and sequenced a new member of each of the four AP subunit families. We have shown by a combination of coimmunoprecipitation and yeast two-hybrid analysis that these four proteins (epsilon, beta4, mu4, and sigma4) are components of a novel adaptor-like heterotetrameric complex, which we are calling AP-4. Immunofluorescence reveals that AP-4 is localized to approximately 10-20 discrete dots in the perinuclear region of the cell. This pattern is disrupted by treating the cells with brefeldin A, indicating that, like other coat proteins, the association of AP-4 with membranes is regulated by the small GTPase ARF. Immunogold electron microscopy indicates that AP-4 is associated with nonclathrin-coated vesicles in the region of the trans-Golgi network. The mu4 subunit of the complex specifically interacts with a tyrosine-based sorting signal, indicating that, like the other three AP complexes, AP-4 is involved in the recognition and sorting of cargo proteins with tyrosine-based motifs. AP-4 is of relatively low abundance, but it is expressed ubiquitously, suggesting that it participates in a specialized trafficking pathway but one that is required in all cell types.
Figures
Figure 1
Sequences of the novel adaptor subunit–related proteins and comparison with their counterparts in AP-1, AP-2, and AP-3. (A) cDNAs encoding the four proteins were originally identified as ESTs encoding homologues of the known adaptor subunits. The complete sequences of the human ε, mouse β4, and human μ4 subunits were obtained by library screening, whereas a full-length human ς4 clone was available as an EST. These sequence data are available from GenBank/EMBL/DDBJ under accession numbers AF155156 (ε), AF155157 (β4), AF155158 (μ4), and AF155159 (ς4). μ4 has been identified previously as μ-adaptin–related protein 2 (μARP2 [Wang and Kilimann, 1997]). (B) The sequences of ε, β4, μ4, and ς4 were compared with the sequences of their counterparts in the AP-1 (left), AP-2 (middle), and AP-3 (right) complexes using the SIP program (Staden, 1990), which was also used to calculate the percent amino acid identity. The novel proteins are no more like any one subunit than the others, indicating that they are novel homologues of the known subunits rather than isoforms. (C) The four members of the γ/α/δ/ε, β, and μ families are shown diagrammatically, together with the positions of some of the conserved motifs: the KRIGYL (K) and WIIGEY (W) motifs in γ, α, δ, and ε; the KKLVYLY (K) and WIIGEY (W) motifs in the β family; and the YELLDE (Y) motif in the μ family.
Figure 2
Expression patterns of ε and β4. Multiple tissue human Northern blots were probed with cDNAs specific for the two novel adaptor subunit–related proteins. Both genes appear to be expressed ubiquitously, but labeling is relatively weak, suggesting that the mRNAs are of low abundance. skel. musc., skeletal muscle.
Figure 3
The four proteins are subunits of a novel adaptor-related complex not associated with clathrin. (A) Pig brain cytosol was immunoprecipitated under nondenaturing conditions using affinity-purified antibodies raised against ε, β4, and μ4 and against the γ subunit of the AP-1 complex. The samples were separated by SDS-PAGE and blotted. The appropriate region of the gel was cut out and probed with ε, β4, and γ antibodies. The results show that the novel proteins, ε, β4 and μ4, all coimmunoprecipitate but do not associate with the γ subunit of the AP-1 complex. (B) Interactions between the four novel proteins were investigated using the yeast two-hybrid system. Yeast cells were transformed with pairs of constructs containing ε, β4, μ4, or ς4 fused with either the transcriptional activation domain (in pGAD424) or the DNA-binding domain (in pGBT9) of the GAL4 promoter. As controls, various combinations of constructs containing subunits of AP-1 and AP-2 complexes were transformed with either ε, β4, μ4, or ς4. The β + μ filters were incubated with substrate for 24 h, whereas the ε + ς filters were incubated with substrate for 8 h. Results show that the combinations of β4 and μ4 and of ε and ς4 interact with each other in a specific manner to produce β-galactosidase activity. No interaction can be detected in alternative pairings with subunits in AP-1 and AP-2 complexes. (C) AP-4 is not enriched in purified clathrin-coated vesicles. Equal protein loadings of clathrin-coated vesicles purified from pig brain and a crude microsomal fraction from a previous stage in the preparation were subjected to SDS-PAGE followed by Western blotting. The blot shows that the γ subunit of AP-1 and the ς2 subunit of AP-2 are strongly enriched in the clathrin-coated vesicles but the μ4 subunit of AP-4 is not.
Figure 4
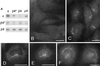
Expression and localization of epitope-tagged β4. (A) β4 tagged with an epitope derived from a neuronal-specific variant of the α subunit of AP-2 was stably transfected into Rat 1 cells. Cytosol from these cells was immunoprecipitated under nondenaturing conditions using affinity-purified antibodies raised against ε, β4, and μ4 and an antibody against the epitope tag that only recognizes the transfected β4 (β4*). The samples were subjected to SDS-PAGE and blotted, and the appropriate region of the blot was cut out and probed with antibodies against ε, tagged β4 (β4*), and total β4. The blot shows that antibodies against ε, β4, and μ4 all bring down tagged β4 as well as endogenous β4, whereas the antibody against the tag brings down ε as well as tagged β4. (B and C) Both control nontransfected cells (B) and cells stably transfected with tagged β4 (C) were labeled for immunofluorescence with a rabbit antibody against the epitope tag. The tagged β4 is localized to a fine pattern of discrete dots in the perinuclear region. This pattern is not seen in the nontransfected cells. (D and E) Stably transfected Rat 1 cells were double labeled with a rabbit antibody against the AP-4 ε subunit (D) and a mouse antibody against the epitope tag (E). Although there is more background than with the rabbit antibody against the tag, the same fine pattern of dots is seen with both antibodies. (F) Nontransfected HeLa cells were labeled with rabbit anti-ε. Again, a fine perinuclear pattern of dots is visible. Bars, 20 μm.
Figure 5
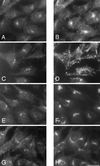
Immunofluorescent double labeling of the tagged β4 subunit of the AP-4 complex with markers for the endocytic and exocytic pathways. Stably transfected Rat 1 cells were double labeled with an antibody against the epitope tag (A, C, E, and G) together with antibodies against the transferrin receptor (B), lgp120 (D), mannosidase II (F), and TGN38 (H). Although all the antibodies give perinuclear labeling, the patterns are distinct from that seen with the antibody against the epitope-tagged β4. However, both mannosidase II and TGN38 are in the same location as the tagged β4, suggesting that AP-4 may be associated with one or both of these compartments. Bar, 20 μm.
Figure 6
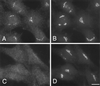
Effect of brefeldin A on the distribution of AP-4. Rat 1 cells stably expressing epitope-tagged β4 were treated with (C and D) or without (A and B) 10 μg/ml brefeldin A for 2 min at 37°C, fixed, and double labeled with antibodies against tagged β4 (A and C) and the Golgi membrane protein mannosidase II (B and D). A complete redistribution of AP-4 can be seen, whereas the mannosidase II labeling is unaffected at this time point. Bar, 20 μm.
Figure 7
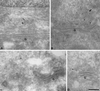
EM localization of AP-4. Stably transfected Rat 1 cells expressing epitope-tagged β4 were labeled with an antibody against the epitope tag followed by protein A coupled to 15-nm colloidal gold. Although the labeling was very low density, consistent with the immunofluorescent results shown in Figures 4–6, transfected cells had more than twofold higher labeling of membranes in the vicinity of the Golgi (G) stack than did nontransfected control cells. (A–D) Four examples of labeled membranes are shown. The label is associated with tubulovesicular membranes, which are likely to correspond to the TGN, but not with the Golgi stack itself. Clathrin-coated buds are frequently seen in the same general area (arrowhead in B), but the AP-4 does not appear to be clathrin associated. Bar, 200 nm.
Figure 8
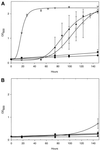
The AP-4 μ4 subunit interacts with a YXXØ motif in a tyrosine-dependent manner. Yeast cells were cotransformed with a vector containing LexA fused with the cytoplasmic domain of wild-type CD63 (KSIRSGYEVM) (A) or a tyrosine-to-alanine mutant (KSIRSGAEVM) (B) and a vector containing VP16 fused to μ1 (●), μ2 (▴), μ3A (▿), or μ4 (○) or a VP16-only control (▪). Cultures were set up containing 0.15 OD600 units of cells in 2 ml of selective medium lacking histidine and grown at 30°C with shaking. OD600 readings were taken between 0 and 148 h to monitor growth in the absence of histidine, which only occurs if the two fusion proteins interact. Each time point represents the mean ± SEM of three separate cultures. (A) μ3A, μ2, and μ4 all interact with the wild-type tail, as measured by the ability of the cells coexpressing the two fusion proteins to grow. (B) This interaction is specific, because mutating the tyrosine to an alanine abolishes growth.
Figure 9
The phylogenetic relationships of subunits of the four AP complexes. A progressive alignment of the amino acid sequences of the four members of the γ/α/δ/ε, β, μ, and ς families was performed using the Clustal method. In every case, the AP-3 subunit appears to have branched off first, followed by the AP-4 subunit and then the AP-1 and AP-2 subunits.
Figure 10
Immunofluorescence labeling comparing the distribution of the four AP complexes in Rat 1 cells. AP-4 is most closely associated with the perinuclear region of the cell, followed by AP-1 and AP-3, whereas AP-2 shows no perinuclear localization because it is associated with the plasma membrane. All four complexes have a punctate pattern, consistent with their association with buds and vesicles; however, there are many fewer dots seen with anti-AP-4 than with antibodies against the other three complexes. Bar, 20 μm.
Similar articles
- AP-4, a novel protein complex related to clathrin adaptors.
Dell'Angelica EC, Mullins C, Bonifacino JS. Dell'Angelica EC, et al. J Biol Chem. 1999 Mar 12;274(11):7278-85. doi: 10.1074/jbc.274.11.7278. J Biol Chem. 1999. PMID: 10066790 - Signal-binding specificity of the mu4 subunit of the adaptor protein complex AP-4.
Aguilar RC, Boehm M, Gorshkova I, Crouch RJ, Tomita K, Saito T, Ohno H, Bonifacino JS. Aguilar RC, et al. J Biol Chem. 2001 Apr 20;276(16):13145-52. doi: 10.1074/jbc.M010591200. Epub 2001 Jan 3. J Biol Chem. 2001. PMID: 11139587 - A late Golgi sorting function for Saccharomyces cerevisiae Apm1p, but not for Apm2p, a second yeast clathrin AP medium chain-related protein.
Stepp JD, Pellicena-Palle A, Hamilton S, Kirchhausen T, Lemmon SK. Stepp JD, et al. Mol Biol Cell. 1995 Jan;6(1):41-58. doi: 10.1091/mbc.6.1.41. Mol Biol Cell. 1995. PMID: 7749194 Free PMC article. - VHS domain -- a longshoreman of vesicle lines.
Lohi O, Poussu A, Mao Y, Quiocho F, Lehto VP. Lohi O, et al. FEBS Lett. 2002 Feb 20;513(1):19-23. doi: 10.1016/s0014-5793(01)03287-2. FEBS Lett. 2002. PMID: 11911875 Review. - The AP-3 complex: a coat of many colours.
Odorizzi G, Cowles CR, Emr SD. Odorizzi G, et al. Trends Cell Biol. 1998 Jul;8(7):282-8. doi: 10.1016/s0962-8924(98)01295-1. Trends Cell Biol. 1998. PMID: 9714600 Review.
Cited by
- Chemical-genetic disruption of clathrin function spares adaptor complex 3-dependent endosome vesicle biogenesis.
Zlatic SA, Grossniklaus EJ, Ryder PV, Salazar G, Mattheyses AL, Peden AA, Faundez V. Zlatic SA, et al. Mol Biol Cell. 2013 Aug;24(15):2378-88. doi: 10.1091/mbc.E12-12-0860. Epub 2013 Jun 12. Mol Biol Cell. 2013. PMID: 23761069 Free PMC article. - Distinct and redundant functions of mu1 medium chains of the AP-1 clathrin-associated protein complex in the nematode Caenorhabditis elegans.
Shim J, Sternberg PW, Lee J. Shim J, et al. Mol Biol Cell. 2000 Aug;11(8):2743-56. doi: 10.1091/mbc.11.8.2743. Mol Biol Cell. 2000. PMID: 10930467 Free PMC article. - GGA proteins associate with Golgi membranes through interaction between their GGAH domains and ADP-ribosylation factors.
Takatsu H, Yoshino K, Toda K, Nakayama K. Takatsu H, et al. Biochem J. 2002 Jul 15;365(Pt 2):369-78. doi: 10.1042/BJ20020428. Biochem J. 2002. PMID: 11950392 Free PMC article. - Altered distribution of ATG9A and accumulation of axonal aggregates in neurons from a mouse model of AP-4 deficiency syndrome.
De Pace R, Skirzewski M, Damme M, Mattera R, Mercurio J, Foster AM, Cuitino L, Jarnik M, Hoffmann V, Morris HD, Han TU, Mancini GMS, Buonanno A, Bonifacino JS. De Pace R, et al. PLoS Genet. 2018 Apr 26;14(4):e1007363. doi: 10.1371/journal.pgen.1007363. eCollection 2018 Apr. PLoS Genet. 2018. PMID: 29698489 Free PMC article. - Frailty in middle age is associated with frailty status and race-specific changes to the transcriptome.
Prince CS, Noren Hooten N, Mode NA, Zhang Y, Ejiogu N, Becker KG, Zonderman AB, Evans MK. Prince CS, et al. Aging (Albany NY). 2019 Aug 8;11(15):5518-5534. doi: 10.18632/aging.102135. Epub 2019 Aug 8. Aging (Albany NY). 2019. PMID: 31395793 Free PMC article.
References
- Ball CL, Hunt SP, Robinson MS. Expression and localization of α-adaptin isoforms. J Cell Sci. 1995;108:2865–2875. - PubMed
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. - PubMed
- Cowles CR, Odorizzi G, Payne GS, Emr SD. The AP-3 adaptor complex is essential for cargo-selective transport to the yeast vacuole. Cell. 1997;91:109–118. - PubMed
- Dell’Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 complex with clathrin. Science. 1998;280:431–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous