Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53 - PubMed (original) (raw)
Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53
M W Halterman et al. J Neurosci. 1999.
Abstract
Hypoxia-induced delayed neuronal death is known to require de novo gene expression; however, the molecular mediators that are involved remain undefined. The transcription factor hypoxia-inducible factor-1alpha (HIF-1alpha), in addition to promoting the expression of adaptive genes under conditions of hypoxia, has been implicated as being a necessary component in p53-mediated cell death in tumors. Using herpes amplicon-mediated gene transfer in cortical neuronal cultures, we demonstrate that delivery of a dominant-negative form of HIF-1alpha (HIFdn), capable of disrupting hypoxia-dependent transcription, reduces delayed neuronal death that follows hypoxic stress. In contrast, hypoxia-resistant p53-null primary cultures are not protected by HIFdn expression. These data indicate that, in hypoxic neurons, HIF-1alpha and p53 conspire to promote a pathological sequence resulting in cell death.
Figures
Fig. 1.
HIF-1α activity in human neuroblastoma lines and cultured mouse primary cortical neurons. A, Normoxic cultured lines and primary neurons express HIF-1α mRNA. Total RNA was harvested from the cell lines Hep3B (lane 1), SHEP-1 (lane 2), SY5Y (lane 3), and mouse cortical neurons (lane 4). Samples (20 μg) were probed by using human and mouse HIF-1α cDNA fragments as described in Materials and Methods. B, HIF-1-responsive reporter plasmids are activated in hypoxic human neuroblastoma lines. SHEP-1 (SH), SY5Y (SY), and Hep3B (HB) cell lines were transfected with the HRE-containing reporter plasmid 18–123F-pXP2 and exposed to normoxic (21% O2) or hypoxic (1% O2) conditions. Then 36 hr later the lysates were tested for hypoxia-dependent reporter activation by luciferase assay. Results from quadruplicate samples are presented in absolute light units × 1000 (mean ± SD).
Fig. 2.
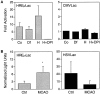
Middle cerebral artery occlusion (MCAO) induces expression from the HIF-1-responsive amplicon reporter HREprLac.A, Hep3B cultures were infected with HREprLac containing herpes particles at an MOI of 0.1 with HREprLac or CMVLac and exposed to 100 μ
m
cobalt chloride (Co), 100 μ
m
desferrioxamine (Df), hypoxia (H; 1% O2) or to hypoxia plus 5 μ
m
DPI (H+DPI) for 36 hr. Quadruplicate samples were assayed for β-galactosidase activity. Data are presented as the fold activation of hypoxic samples relative to the average normoxic untreated control (mean ± SD). _B,_HIF-1-responsive reporter expression is increased in the ischemic cortex. At 4 d after the delivery of HREprLac and HSVLac virus, the mice were subjected to middle cerebral artery occlusion. Then 24 hr later the injected coronal sections were harvested and analyzed as described in Materials and Methods. Values represent β-galactosidase activity per unit of viral genome recovered (mean ± SD) for control (n = 3) and stroked (_n_= 5) mice (*p < 0.05 vs nonischemic control by unpaired two-tailed t test).
Fig. 3.
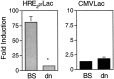
HIFdn attenuates expression from a HIF-1-sensitive reporter plasmid. Hep3B cells were transiently transfected with the reporter plasmids HRE2prLac or CMVLac and either HIFdn (dn) or pBS (BS). Monolayers were exposed to control (21% O2) or hypoxic (1% O2) conditions for 36 hr and analyzed for β-galactosidase activity. Data are presented as the fold induction of quadruplicate hypoxic samples (mean ± SD) relative to normoxic transfected controls (*p < 0.001 by unpaired two-tailed t test).
Fig. 4.
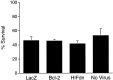
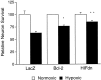
Expression of Bcl-2 or HIFdn does not protect cortical neurons from rapid cell death. Mixed cortical cultures (DIV 16–19) were infected with HSV amplicon viruses expressing β-galactosidase (LacZ), Bcl-2, or HIFdn and allowed to incubate under normoxic conditions for 20 hr. Cultures were exposed to hypoxia (0.5%) in EBSS (1.1 m
m
glucose) for 4 hr, returned to normoxic–normoglycemic conditions, and assessed for viability 24 hr later by trypan blue exclusion. Data are expressed as the absolute percentage of surviving neurons (live/total) from infected and uninfected hypoxic wells (mean ± SD; based on an average of five measurements made from triplicate wells).
Fig. 5.
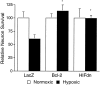
Disruption of HIF-1 signaling protects hypoxic primary cortical neurons from delayed death. Mixed cortical cultures (DIV 9–12) were infected with HSV amplicon viruses expressing β-galactosidase, Bcl-2_,_ or HIFdn and were allowed to express for 20 hr. Cultures were exposed to hypoxia (0.5%) in glucose-free EBSS for 110 min in the presence of 10 μ
m
MK-801 and 100 μ
m
CNQX and returned to normoxic conditions. Cultures were refed with fresh EBSS (25 m
md
-glucose) and assessed 46 hr later by trypan blue exclusion. Data are expressed as the percentage of surviving hypoxic neurons relative to the survival of normoxic infected wells set to 100% (mean ± SD; *p < 0.0001 vs hypoxic LacZ control by unpaired two-tailed t test).
Fig. 6.
HIFdn protects hypoxic purified cortical neurons from delayed death. Cortical neurons, cultured in B27-supplemented Neurobasal medium, were infected with β-galactosidase, Bcl-2, or HIFdn-expressing amplicon viruses and allowed to express for 20 hr. Cultures were exposed to hypoxia (0.5%) in glucose-free EBSS for 110 min in the presence of 10 μ
m
MK-801 and 100 μ
m
CNQX and returned to normoxic conditions. Cultures were refed with fresh EBSS (25 m
md
-glucose) and assessed for viability 46 hr later by trypan blue exclusion. Data are expressed as the percentage of surviving hypoxic neurons relative to the survival of normoxic infected wells set to 100% (mean ± SD; *p < 0.01, **p < 0.001 vs hypoxic LacZ control by unpaired two-tailed _t_test).
Fig. 7.
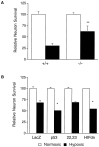
HIF-1α promotes delayed neuronal death in a p53-dependent manner. A, p53-Deficient neurons are resistant to hypoxia-induced delayed neuronal death. Uninfected, wild-type (+/+), or p53 homozygous null (−/−) mixed cortical cultures were exposed to hypoxia (0.5%) in glucose-free EBSS for 110 min in the presence of 10 μ
m
MK-801 and 100 μ
m
CNQX and returned to normoxic conditions. Cultures were refed with fresh EBSS (25 m
md
-glucose) and assessed for viability 46 hr later by trypan blue exclusion. Data are expressed as the percentage of surviving hypoxic wild-type or knock-out neurons relative to normoxic controls set to 100% (mean ± SD; **p < 0.0001 vs hypoxic wild-type cultures by unpaired two-tailed t test). _B,Protection from delayed death by HIF-1 dominant-negative disruption requires p53. p53-Deficient mixed primary cultures were infected at an MOI of 1.25, with amplicon viruses expressing β-galactosidase (LacZ), p53, the mutant p5322,23, or HIFdn. Infected cultures were exposed to the delayed death paradigm and analyzed for survival as described in Figure 7_A(mean ± SD; *p < 0.05 comparing p53-, p5322,23-, and HIFdn-infected cultures against hypoxic HSVLac-infected cultures).
Similar articles
- HIF-1alpha and p53 promote hypoxia-induced delayed neuronal death in models of CNS ischemia.
Halterman MW, Federoff HJ. Halterman MW, et al. Exp Neurol. 1999 Sep;159(1):65-72. doi: 10.1006/exnr.1999.7160. Exp Neurol. 1999. PMID: 10486175 Review. - Inhibition of hypoxia inducible factor 1alpha causes oxygen-independent cytotoxicity and induces p53 independent apoptosis in glioblastoma cells.
Dai S, Huang ML, Hsu CY, Chao KS. Dai S, et al. Int J Radiat Oncol Biol Phys. 2003 Mar 15;55(4):1027-36. doi: 10.1016/s0360-3016(02)04507-8. Int J Radiat Oncol Biol Phys. 2003. PMID: 12605983 - Up-regulation of hypoxia-inducible factor-1alpha is not sufficient for hypoxic/anoxic p53 induction.
Wenger RH, Camenisch G, Desbaillets I, Chilov D, Gassmann M. Wenger RH, et al. Cancer Res. 1998 Dec 15;58(24):5678-80. Cancer Res. 1998. PMID: 9865721 - Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis.
Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Carmeliet P, et al. Nature. 1998 Jul 30;394(6692):485-90. doi: 10.1038/28867. Nature. 1998. PMID: 9697772 - HIF-1 and p53: communication of transcription factors under hypoxia.
Schmid T, Zhou J, Brüne B. Schmid T, et al. J Cell Mol Med. 2004 Oct-Dec;8(4):423-31. doi: 10.1111/j.1582-4934.2004.tb00467.x. J Cell Mol Med. 2004. PMID: 15601571 Free PMC article. Review.
Cited by
- Melatonin Inhibits Hypoxia-Induced Alzheimer's Disease Pathogenesis by Regulating the Amyloidogenic Pathway in Human Neuroblastoma Cells.
Singrang N, Nopparat C, Panmanee J, Govitrapong P. Singrang N, et al. Int J Mol Sci. 2024 May 10;25(10):5225. doi: 10.3390/ijms25105225. Int J Mol Sci. 2024. PMID: 38791263 Free PMC article. - Molecular Mechanisms of Neuroprotection after the Intermittent Exposures of Hypercapnic Hypoxia.
Tregub PP, Kulikov VP, Ibrahimli I, Tregub OF, Volodkin AV, Ignatyuk MA, Kostin AA, Atiakshin DA. Tregub PP, et al. Int J Mol Sci. 2024 Mar 25;25(7):3665. doi: 10.3390/ijms25073665. Int J Mol Sci. 2024. PMID: 38612476 Free PMC article. Review. - PDGFRβ + cell HIF2α is dispensable for white adipose tissue metabolic remodeling and hepatic lipid accumulation in obese mice.
Yao T, Wei D, Tian X, Zhao L, Wan Q, Zhang X, Cai J, Li S, Diao B, Feng S, Shan B, Shao M, Wu Y. Yao T, et al. Lipids Health Dis. 2024 Mar 20;23(1):81. doi: 10.1186/s12944-024-02069-1. Lipids Health Dis. 2024. PMID: 38509584 Free PMC article. - Nitric Oxide-Dependent Pathways as Critical Factors in the Consequences and Recovery after Brain Ischemic Hypoxia.
Wierońska JM, Cieślik P, Kalinowski L. Wierońska JM, et al. Biomolecules. 2021 Jul 26;11(8):1097. doi: 10.3390/biom11081097. Biomolecules. 2021. PMID: 34439764 Free PMC article. Review. - Adiponectin Treatment Attenuates Cerebral Ischemia-Reperfusion Injury through HIF-1_α_-Mediated Antioxidation in Mice.
Zhang C, Zhen L, Fang Z, Yu L, Zhang Y, Wei H, Jia J, Wang S. Zhang C, et al. Oxid Med Cell Longev. 2021 Jul 14;2021:5531048. doi: 10.1155/2021/5531048. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 34336097 Free PMC article.
References
- Abe K, Setoguchi Y, Hayashi T, Itoyama Y. In vivo adenovirus-mediated gene transfer and the expression in ischemic and reperfused rat brain. Brain Res. 1997;763:191–201. - PubMed
- An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1α. Nature. 1998;392:405–408. - PubMed
- Blagosklonny MV, An WG, Romanova LY, Trepe J, Fojo T, Neckers L. p53 inhibits hypoxia-inducible factor-stimulated transcription. J Biol Chem. 1998;273:11995–11998. - PubMed
- Brewer GJ. Serum-free B27/Neurobasal medium supports differentiated growth of neurons from the striatum, substantia nigra, septum, cerebral cortex, cerebellum, and dentate gyrus. J Neurosci Res. 1995;42:674–683. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F30 MH012305/MH/NIMH NIH HHS/United States
- F30 MH012305-03/MH/NIMH NIH HHS/United States
- HD31300/HD/NICHD NIH HHS/United States
- 1F30MH12305-01/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous