Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4 - PubMed (original) (raw)
. 1999 Oct;19(10):7096-105.
doi: 10.1128/MCB.19.10.7096.
I Shiojima, Y Hiroi, S Kudoh, T Oka, E Takimoto, D Hayashi, T Hosoda, A Habara-Ohkubo, T Nakaoka, T Fujita, Y Yazaki, I Komuro
Affiliations
- PMID: 10490646
- PMCID: PMC84704
- DOI: 10.1128/MCB.19.10.7096
Bone morphogenetic proteins induce cardiomyocyte differentiation through the mitogen-activated protein kinase kinase kinase TAK1 and cardiac transcription factors Csx/Nkx-2.5 and GATA-4
K Monzen et al. Mol Cell Biol. 1999 Oct.
Abstract
Bone morphogenetic proteins (BMPs) have been shown to induce ectopic expression of cardiac transcription factors and beating cardiomyocytes in nonprecardiac mesodermal cells in chicks, suggesting that BMPs are inductive signaling molecules that participate in the development of the heart. However, the precise molecular mechanisms by which BMPs regulate cardiac development are largely unknown. In the present study, we examined the molecular mechanisms by which BMPs induce cardiac differentiation by using the P19CL6 in vitro cardiomyocyte differentiation system, a clonal derivative of P19 embryonic teratocarcinoma cells. We established a permanent P19CL6 cell line, P19CL6noggin, which constitutively overexpresses the BMP antagonist noggin. Although almost all parental P19CL6 cells differentiate into beating cardiomyocytes when treated with 1% dimethyl sulfoxide, P19CL6noggin cells did not differentiate into beating cardiomyocytes nor did they express cardiac transcription factors or contractile protein genes. The failure of differentiation was rescued by overexpression of BMP-2 or addition of BMP protein to the culture media, indicating that BMPs were indispensable for cardiomyocyte differentiation in this system. Overexpression of TAK1, a member of the mitogen-activated protein kinase kinase kinase superfamily which transduces BMP signaling, restored the ability of P19CL6noggin cells to differentiate into cardiomyocytes and concomitantly express cardiac genes, whereas overexpression of the dominant negative form of TAK1 in parental P19CL6 cells inhibited cardiomyocyte differentiation. Overexpression of both cardiac transcription factors Csx/Nkx-2.5 and GATA-4 but not of Csx/Nkx-2.5 or GATA-4 alone also induced differentiation of P19CL6noggin cells into cardiomyocytes. These results suggest that TAK1, Csx/Nkx-2.5, and GATA-4 play a pivotal role in the cardiogenic BMP signaling pathway.
Figures
FIG. 1
Inhibition of cardiomyocyte differentiation by overexpression of noggin. (A) P19CL6 cells and P19CL6noggin cells were cultured in growth medium (a, b, e, and f) or in differentiation medium containing 1% DMSO (c, d, g, and h). Both parental P19CL6 cells (a and b) and P19CL6noggin cells (e and f) grew well and remained undifferentiated in growth medium. However, parental P19CL6 cells differentiated into beating cardiomyocytes when cultured in medium containing 1% DMSO. On day 14, most P19CL6 cells had differentiated into mononucleated contracting cardiomyocytes (c [magnification, × 1,000] and d [magnification, ×200]). On the other hand, P19CL6noggin cells did not differentiate into beating cardiomyocytes after treatment with DMSO (g). Overexpression of BMP-2 with adenovirus induced differentiation of P19CL6noggin cells into cardiomyocytes (h), suggesting the essential role of BMPs in the differentiation of P19CL6. The cells were stained with anti-sarcomeric MHC antibody (MF20) (a, c to e, g, and h) or Hoechst dye (b and f). (B) Quantification of the areas stained by MF20 in P19CL6 and P19CL6noggin cells. Like overexpression of BMP-2 with adenovirus, addition of BMP protein to the culture medium at the concentration of 100 ng/ml but not 10 ng/ml partially induced differentiation of P19CL6noggin cells into cardiomyocytes (columns 5 to 7). The areas of at least five fields were measured for each cell line under the same conditions. The results are expressed as the mean percents ± standard deviations.
FIG. 1
Inhibition of cardiomyocyte differentiation by overexpression of noggin. (A) P19CL6 cells and P19CL6noggin cells were cultured in growth medium (a, b, e, and f) or in differentiation medium containing 1% DMSO (c, d, g, and h). Both parental P19CL6 cells (a and b) and P19CL6noggin cells (e and f) grew well and remained undifferentiated in growth medium. However, parental P19CL6 cells differentiated into beating cardiomyocytes when cultured in medium containing 1% DMSO. On day 14, most P19CL6 cells had differentiated into mononucleated contracting cardiomyocytes (c [magnification, × 1,000] and d [magnification, ×200]). On the other hand, P19CL6noggin cells did not differentiate into beating cardiomyocytes after treatment with DMSO (g). Overexpression of BMP-2 with adenovirus induced differentiation of P19CL6noggin cells into cardiomyocytes (h), suggesting the essential role of BMPs in the differentiation of P19CL6. The cells were stained with anti-sarcomeric MHC antibody (MF20) (a, c to e, g, and h) or Hoechst dye (b and f). (B) Quantification of the areas stained by MF20 in P19CL6 and P19CL6noggin cells. Like overexpression of BMP-2 with adenovirus, addition of BMP protein to the culture medium at the concentration of 100 ng/ml but not 10 ng/ml partially induced differentiation of P19CL6noggin cells into cardiomyocytes (columns 5 to 7). The areas of at least five fields were measured for each cell line under the same conditions. The results are expressed as the mean percents ± standard deviations.
FIG. 2
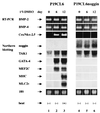
Expression of cardiac-specific genes was detected in P19CL6 cells but not in P19CL6noggin cells. RNA was prepared from parental P19CL6 cells and P19CL6noggin cells on day 0 (before treatment with DMSO) (lanes 1 and 4), day 6 (lanes 2 and 5), and day 12 (lanes 3 and 6). RT-PCR was performed to analyze BMP-2, BMP-4, and Csx/Nkx-2.5 mRNA. Ten micrograms of RNA from each sample was subjected to Northern blot analysis for other genes. Ethidium bromide staining of rRNA is presented at the bottom to show that the same amount of intact RNA was loaded in each lane.
FIG. 3
Overexpression of TAK1 induced the differentiation of P19CL6noggin cells into cardiomyocytes. (A) Expression plasmids containing the murine TAK1 gene and their mutants were transfected into P19CL6 and P19CL6noggin cells on day 2 of differentiation by the lipofection method. Compared with untransfected control P19CL6 cells (a), the P19CL6 cells transfected with dnTAK1 differentiated into MF20-positive cardiomyocytes less efficiently (b). In contrast to control P19CL6noggin cells (c), the P19CL6noggin cells transfected with wild-type TAK1 (d) and caTAK1 (e) partially differentiated into beating cardiomyocytes. The cells transfected with dnTAK1 (f) did not differentiate into beating cardiomyocytes. The cells were stained with MF20 on day 14. (B) Quantification of the areas stained by MF20 in P19CL6 and P19CL6noggin cells. The areas of at least five fields were measured for each cell line under the same conditions. The results are expressed as mean percents ± standard deviations.
FIG. 3
Overexpression of TAK1 induced the differentiation of P19CL6noggin cells into cardiomyocytes. (A) Expression plasmids containing the murine TAK1 gene and their mutants were transfected into P19CL6 and P19CL6noggin cells on day 2 of differentiation by the lipofection method. Compared with untransfected control P19CL6 cells (a), the P19CL6 cells transfected with dnTAK1 differentiated into MF20-positive cardiomyocytes less efficiently (b). In contrast to control P19CL6noggin cells (c), the P19CL6noggin cells transfected with wild-type TAK1 (d) and caTAK1 (e) partially differentiated into beating cardiomyocytes. The cells transfected with dnTAK1 (f) did not differentiate into beating cardiomyocytes. The cells were stained with MF20 on day 14. (B) Quantification of the areas stained by MF20 in P19CL6 and P19CL6noggin cells. The areas of at least five fields were measured for each cell line under the same conditions. The results are expressed as mean percents ± standard deviations.
FIG. 4
Cardiac-specific genes were expressed in P19CL6noggin cells overexpressing TAK1. RNA was extracted from untransfected parental P19CL6 cells and the P19CL6noggin cells transfected with TAK1 and their mutants on day 14. RT-PCR was performed for the analysis of Csx/Nkx-2.5 mRNA. Ten micrograms of RNA from each sample was subjected to Northern blot analysis for other genes. Ethidium bromide staining of rRNA is presented at the bottom to show that the same amount of intact RNA was loaded in each lane.
FIG. 5
Simultaneous overexpression of Csx/Nkx-2.5 and GATA-4 but not of Csx/Nkx-2.5 or GATA-4 alone induced differentiation of P19CL6noggin cells into cardiomyocytes. (A) Expression plasmids containing human CSX1a cDNA or murine GATA-4 cDNA were transfected into P19CL6noggin cells on day 2 by the lipofection method. Like untransfected control P19CL6noggin cells (a), the P19CL6noggin cells overexpressing Csx/Nkx-2.5 alone (b) or GATA-4 alone (c) did not differentiate into beating cardiomyocytes, whereas simultaneous overexpression of both Csx/Nkx-2.5 and GATA-4 in P19CL6noggin cells markedly induced their differentiation into cardiomyocytes (d). The cells were stained with MF20 on day 14. (B) Quantification of the areas stained by MF20 in P19CL6noggin cells. The areas of at least five fields were measured for each cell line under the same conditions. The results are expressed as mean percents ± standard deviations.
FIG. 5
Simultaneous overexpression of Csx/Nkx-2.5 and GATA-4 but not of Csx/Nkx-2.5 or GATA-4 alone induced differentiation of P19CL6noggin cells into cardiomyocytes. (A) Expression plasmids containing human CSX1a cDNA or murine GATA-4 cDNA were transfected into P19CL6noggin cells on day 2 by the lipofection method. Like untransfected control P19CL6noggin cells (a), the P19CL6noggin cells overexpressing Csx/Nkx-2.5 alone (b) or GATA-4 alone (c) did not differentiate into beating cardiomyocytes, whereas simultaneous overexpression of both Csx/Nkx-2.5 and GATA-4 in P19CL6noggin cells markedly induced their differentiation into cardiomyocytes (d). The cells were stained with MF20 on day 14. (B) Quantification of the areas stained by MF20 in P19CL6noggin cells. The areas of at least five fields were measured for each cell line under the same conditions. The results are expressed as mean percents ± standard deviations.
FIG. 6
Expression of cardiac-specific genes in P19CL6noggin cells overexpressing Csx/Nkx-2.5 and/or GATA-4. RNA was extracted from parental P19CL6 cells and the P19CL6noggin cells transfected with Csx/Nkx-2.5 and/or GATA-4 on day 14. RT-PCR was performed for the analysis of Csx/Nkx-2.5 mRNA. Ten micrograms of RNA from each sample was subjected to Northern blot analysis for other genes. Ethidium bromide staining of rRNA is presented at the bottom to show that the same amount of intact RNA was loaded in each lane.
FIG. 7
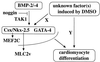
Speculative diagram of the regulatory network controlling differentiation of P19CL6 into cardiomyocytes. Initially, BMPs (especially BMP-2 and BMP-4) and an unknown factor(s) induced by DMSO cooperatively transactivate the expression of Csx/Nkx-2.5 and GATA-4. This transactivation is inhibited by a BMP antagonist noggin and mediated at least by a MAPKKK family TAK1. Subsequently, Csx/Nkx-2.5 and GATA-4 cooperatively function to induce terminal differentiation into cardiomyocytes. The unknown factor(s) induced by DMSO is also required for this step, because neither expression of Csx/Nkx-2.5 and GATA-4 nor subsequent terminal differentiation into cardiomyocytes was induced in the absence of DMSO. Although some cardiac-specific genes, such as MEF2C and MLC2v, are upregulated by Csx/Nkx-2.5 alone, differentiation into beating cardiomyocytes requires the cooperative effects of both Csx/Nkx-2.5 and GATA-4.
Similar articles
- Smads, TAK1, and their common target ATF-2 play a critical role in cardiomyocyte differentiation.
Monzen K, Hiroi Y, Kudoh S, Akazawa H, Oka T, Takimoto E, Hayashi D, Hosoda T, Kawabata M, Miyazono K, Ishii S, Yazaki Y, Nagai R, Komuro I. Monzen K, et al. J Cell Biol. 2001 May 14;153(4):687-98. doi: 10.1083/jcb.153.4.687. J Cell Biol. 2001. PMID: 11352931 Free PMC article. - Early stage-specific inhibitions of cardiomyocyte differentiation and expression of Csx/Nkx-2.5 and GATA-4 by phosphatidylinositol 3-kinase inhibitor LY294002.
Naito AT, Tominaga A, Oyamada M, Oyamada Y, Shiraishi I, Monzen K, Komuro I, Takamatsu T. Naito AT, et al. Exp Cell Res. 2003 Nov 15;291(1):56-69. doi: 10.1016/s0014-4827(03)00378-1. Exp Cell Res. 2003. PMID: 14597408 - Regulation of MAP kinase by the BMP-4/TAK1 pathway in Xenopus ectoderm.
Goswami M, Uzgare AR, Sater AK. Goswami M, et al. Dev Biol. 2001 Aug 15;236(2):259-70. doi: 10.1006/dbio.2001.0338. Dev Biol. 2001. PMID: 11476570 - A role for bone morphogenetic protein signaling in cardiomyocyte differentiation.
Monzen K, Nagai R, Komuro I. Monzen K, et al. Trends Cardiovasc Med. 2002 Aug;12(6):263-9. doi: 10.1016/s1050-1738(02)00172-x. Trends Cardiovasc Med. 2002. PMID: 12242050 Review. - GATA transcription factors and cardiac development.
Charron F, Nemer M. Charron F, et al. Semin Cell Dev Biol. 1999 Feb;10(1):85-91. doi: 10.1006/scdb.1998.0281. Semin Cell Dev Biol. 1999. PMID: 10355032 Review.
Cited by
- Pitx2-mediated cardiac outflow tract remodeling.
Ma HY, Xu J, Eng D, Gross MK, Kioussi C. Ma HY, et al. Dev Dyn. 2013 May;242(5):456-68. doi: 10.1002/dvdy.23934. Epub 2013 Mar 12. Dev Dyn. 2013. PMID: 23361844 Free PMC article. - Jarid2 is among a set of genes differentially regulated by Nkx2.5 during outflow tract morphogenesis.
Barth JL, Clark CD, Fresco VM, Knoll EP, Lee B, Argraves WS, Lee KH. Barth JL, et al. Dev Dyn. 2010 Jul;239(7):2024-33. doi: 10.1002/dvdy.22341. Dev Dyn. 2010. PMID: 20549724 Free PMC article. - Sox6 regulation of cardiac myocyte development.
Cohen-Barak O, Yi Z, Hagiwara N, Monzen K, Komuro I, Brilliant MH. Cohen-Barak O, et al. Nucleic Acids Res. 2003 Oct 15;31(20):5941-8. doi: 10.1093/nar/gkg807. Nucleic Acids Res. 2003. PMID: 14530442 Free PMC article. - NEXN inhibits GATA4 and leads to atrial septal defects in mice and humans.
Yang F, Zhou L, Wang Q, You X, Li Y, Zhao Y, Han X, Chang Z, He X, Cheng C, Wu C, Wang WJ, Hu FY, Zhao T, Li Y, Zhao M, Zheng GY, Dong J, Fan C, Yang J, Meng X, Zhang Y, Zhu X, Xiong J, Tian XL, Cao H. Yang F, et al. Cardiovasc Res. 2014 Jul 15;103(2):228-37. doi: 10.1093/cvr/cvu134. Epub 2014 May 27. Cardiovasc Res. 2014. PMID: 24866383 Free PMC article. - Cardiomyogenic stem and progenitor cell plasticity and the dissection of cardiopoiesis.
Perino MG, Yamanaka S, Li J, Wobus AM, Boheler KR. Perino MG, et al. J Mol Cell Cardiol. 2008 Oct;45(4):475-94. doi: 10.1016/j.yjmcc.2008.05.002. Epub 2008 May 11. J Mol Cell Cardiol. 2008. PMID: 18565538 Free PMC article. Review.
References
- Andrée B, Duprez D, Vorbusch B, Arnold H, Brand T. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech Dev. 1998;70:119–131. - PubMed
- Bodmer R, Jan L Y, Jan Y N. A new homeobox-containing gene, msh-2, is transiently expressed early during mesoderm formation in Drosophila. Development. 1990;110:661–669. - PubMed
- Bodmer R. The gene tinman is required for specification of the heart and visceral muscles in Drosophila. Development. 1993;118:719–729. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous