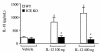IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor - PubMed (original) (raw)
IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor
G Fantuzzi et al. J Clin Invest. 1999 Sep.
Abstract
IL-12 and IL-18 are IFN-gamma-inducing cytokines. In the present study, the role of endogenous IL-18 in the induction of IFN-gamma by IL-12 was investigated in mice. In the presence of a specific inhibitor of caspase-1 (also known as IL-1beta-converting enzyme, or ICE) IL-12-induced IFN-gamma from splenocytes was reduced by 85%. Using splenocytes from ICE-deficient mice, IL-12-induced IFN-gamma was reduced by 80%. However, the role of ICE was not through processing and release of IL-1beta. Neutralizing anti-IL-18 IgG reduced IL-12-induced IFN-gamma in splenocytes by 85%. Splenocytes cultured in vitro spontaneously released IL-18 into the extracellular compartment over time. Extracellular levels of IL-18 significantly correlated with IL-12-induced IFN-gamma and were reduced in cells obtained from ICE-deficient mice. In vivo, IL-12 administration increased circulating levels of IL-18 in wild-type mice but not in ICE-deficient mice. Both neutralization of IL-18 and ICE deficiency significantly reduced induction of circulating IFN-gamma in mice receiving IL-12. The IL-18 precursor was constitutively expressed in the livers and spleens of untreated mice. Furthermore, administration of IL-12 significantly increased liver-associated IL-18 levels. These data demonstrate that endogenous, ICE-cleaved IL-18 significantly contributes to induction of IFN-gamma by IL-12.
Figures
Figure 1
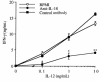
Effect of IL-18 neutralization on the induction of IFN-γ by IL-12 in vitro. Splenocytes were cultured for 48 hours with increasing concentrations of IL-12 (0.1–10 ng/mL) in the presence or absence of either neutralizing anti–IL-18 IgG or control IgG (50 μg/mL). IFN-γ levels were measured in the supernatants. Data are mean ± SEM of 3 mice per group and are representative of 1 experiment out of 3 performed. *P < 0.05, **P < 0.01 vs. RPMI or control IgG by repeated-measures ANOVA.
Figure 2
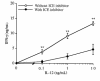
Effect of ICE inhibition on IFN-γ induced by IL-12 in vitro. Splenocytes were cultured for 48 hours with increasing concentrations of IL-12 (0.1–10 ng/mL) in the presence or absence of ICE inhibitor (20 μM). IFN-γ levels were measured in the supernatants. Data are mean ± SEM of 3 mice per group and are representative of 1 experiment out of 3 performed. *P < 0.05, **P < 0.01 by paired Student’s t test.
Figure 3
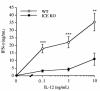
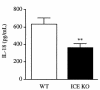
Production of IFN-γ in splenocytes from ICE KO mice. Splenocytes from WT or ICE KO mice were cultured for 48 hours with increasing concentrations of IL-12 (0.1–10 ng/mL). IFN-γ levels were measured in the supernatants. Data are mean ± SEM of 6 mice per group. **P < 0.01, ***P < 0.001 WT vs. ICE KO by unpaired Student’s t test.
Figure 4
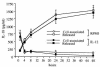
IL-18 production in unstimulated and IL-12–stimulated splenocytes. Splenocytes were cultured in RPMI-FBS for increasing amounts of time without added exogenous stimulation, or with IL-12 at 10 ng/mL. Cell-associated and released IL-18 levels were measured. Data are mean ± SEM of 9 mice per group.
Figure 5
Release of IL-18 in splenocytes from ICE KO mice. Splenocytes from WT or ICE KO mice were cultured for 24 hours in the absence of any exogenous stimulation. IL-18 levels were measured in supernatants. Data are mean ± SEM of 8 mice per group. **P < 0.01 by factorial ANOVA.
Figure 6
Effect of in vivo administration of IL-12 on IFN-γ levels in ICE KO and WT mice. WT and ICE KO mice received 4 daily intraperitoneal injections of IL-12 (100 or 400 ng/mouse) or vehicle. Two hours after the fourth injection, blood was collected and serum was prepared for measurement of IFN-γ. Data are mean ± SEM of 10 mice per group. *P < 0.05 vs. WT mice by factorial ANOVA.
Figure 7
Increase in serum IL-18 levels after IL-12 administration. WT and ICE KO mice received 4 daily intraperitoneal injections of IL-12 (100 or 400 ng/mouse) or vehicle. Two hours after the fourth injection, blood was collected and serum was prepared. Data are mean ± SEM of 10 mice per group. *P < 0.05 vs. WT mice. ‡P < 0.01 vs. corresponding vehicle by factorial ANOVA.
Figure 8
Constitutive expression of pro–IL-18 in the spleens and livers on untreated mice. Spleen and liver homogenates from 3 untreated mice were subjected to Western blot analysis with a specific rabbit anti-murine IL-18 antiserum. Recombinant murine pro–IL-18 and mature IL-18 were used as standards.
Figure 9
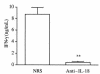
Neutralization of IL-18 reduces IL-12–induced IFN-γ levels in vivo. Mice received 4 daily intraperitoneal injections of IL-12 (100 ng/mouse). One hour before the first and the third injection, the antibody-treated group received an intraperitoneal injection of 200 μL of rabbit anti–IL-18 antiserum, whereas the control group received the same amount of NRS. Two hours after the fourth injection, blood was collected and serum was prepared for measurement of IFN-γ. Data are mean ± SEM of 5 mice per group. **P < 0.01 vs. control by unpaired Student’s t test.
Similar articles
- Caspase-1 processes IFN-gamma-inducing factor and regulates LPS-induced IFN-gamma production.
Ghayur T, Banerjee S, Hugunin M, Butler D, Herzog L, Carter A, Quintal L, Sekut L, Talanian R, Paskind M, Wong W, Kamen R, Tracey D, Allen H. Ghayur T, et al. Nature. 1997 Apr 10;386(6625):619-23. doi: 10.1038/386619a0. Nature. 1997. PMID: 9121587 - The interleukin-1 beta-converting enzyme inhibitor pralnacasan reduces dextran sulfate sodium-induced murine colitis and T helper 1 T-cell activation.
Loher F, Bauer C, Landauer N, Schmall K, Siegmund B, Lehr HA, Dauer M, Schoenharting M, Endres S, Eigler A. Loher F, et al. J Pharmacol Exp Ther. 2004 Feb;308(2):583-90. doi: 10.1124/jpet.103.057059. Epub 2003 Nov 10. J Pharmacol Exp Ther. 2004. PMID: 14610233 - Interleukin-18 and host defense against infection.
Dinarello CA, Fantuzzi G. Dinarello CA, et al. J Infect Dis. 2003 Jun 15;187 Suppl 2:S370-84. doi: 10.1086/374751. J Infect Dis. 2003. PMID: 12792854 Review. - Interleukin-1beta converting enzyme (caspase-1) in intestinal inflammation.
Siegmund B. Siegmund B. Biochem Pharmacol. 2002 Jul 1;64(1):1-8. doi: 10.1016/s0006-2952(02)01064-x. Biochem Pharmacol. 2002. PMID: 12106600 Review.
Cited by
- CpG-induced IFNgamma expands TLR4-specific IL-18 responses in vivo.
Gupta S, Gould MP, DeVecchio J, Canaday DH, Auletta JJ, Heinzel FP. Gupta S, et al. Cell Immunol. 2006 Oct;243(2):75-82. doi: 10.1016/j.cellimm.2006.12.004. Epub 2007 Feb 9. Cell Immunol. 2006. PMID: 17292338 Free PMC article. - Up-regulation of IL-18 and predominance of a Th1 immune response is a hallmark of lupus nephritis.
Calvani N, Richards HB, Tucci M, Pannarale G, Silvestris F. Calvani N, et al. Clin Exp Immunol. 2004 Oct;138(1):171-8. doi: 10.1111/j.1365-2249.2004.02588.x. Clin Exp Immunol. 2004. PMID: 15373921 Free PMC article. - IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease.
Bossù P, Neumann D, Del Giudice E, Ciaramella A, Gloaguen I, Fantuzzi G, Dinarello CA, Di Carlo E, Musiani P, Meroni PL, Caselli G, Ruggiero P, Boraschi D. Bossù P, et al. Proc Natl Acad Sci U S A. 2003 Nov 25;100(24):14181-6. doi: 10.1073/pnas.2336094100. Epub 2003 Nov 13. Proc Natl Acad Sci U S A. 2003. PMID: 14615579 Free PMC article. - The oral histone deacetylase inhibitor ITF2357 reduces cytokines and protects islet β cells in vivo and in vitro.
Lewis EC, Blaabjerg L, Størling J, Ronn SG, Mascagni P, Dinarello CA, Mandrup-Poulsen T. Lewis EC, et al. Mol Med. 2011 May-Jun;17(5-6):369-77. doi: 10.2119/molmed.2010.00152. Epub 2010 Dec 22. Mol Med. 2011. PMID: 21193899 Free PMC article. - Maternal milk regulation of cell infiltration and interleukin 18 in the intestine of suckling rat pups.
Penttila IA, Flesch IE, McCue AL, Powell BC, Zhou FH, Read LC, Zola H. Penttila IA, et al. Gut. 2003 Nov;52(11):1579-86. doi: 10.1136/gut.52.11.1579. Gut. 2003. PMID: 14570726 Free PMC article.
References
- Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–511. - PubMed
- Dinarello CA, et al. Overview of interleukin-18: more than an interferon-γ inducing factor. J Leukoc Biol. 1998;63:658–664. - PubMed
- Sacco S, et al. Protective effect of a single interleukin-12 (IL-12) predose against the toxicity of subsequent chronic IL-12 in mice: role of cytokines and glucocorticoids. Blood. 1997;90:4473–4479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI-15614/AI/NIAID NIH HHS/United States
- R01 AI015614/AI/NIAID NIH HHS/United States
- P30 CA046934/CA/NCI NIH HHS/United States
- R56 AI015614/AI/NIAID NIH HHS/United States
- CA-46934/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous