Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo - PubMed (original) (raw)
Cytoplasmic dynein is required for distinct aspects of MTOC positioning, including centrosome separation, in the one cell stage Caenorhabditis elegans embryo
P Gönczy et al. J Cell Biol. 1999.
Abstract
We have investigated the role of cytoplasmic dynein in microtubule organizing center (MTOC) positioning using RNA-mediated interference (RNAi) in Caenorhabditis elegans to deplete the product of the dynein heavy chain gene dhc-1. Analysis with time-lapse differential interference contrast microscopy and indirect immunofluorescence revealed that pronuclear migration and centrosome separation failed in one cell stage dhc-1 (RNAi) embryos. These phenotypes were also observed when the dynactin components p50/dynamitin or p150(Glued) were depleted with RNAi. Moreover, in 15% of dhc-1 (RNAi) embryos, centrosomes failed to remain in proximity of the male pronucleus. When dynein heavy chain function was diminished only partially with RNAi, centrosome separation took place, but orientation of the mitotic spindle was defective. Therefore, cytoplasmic dynein is required for multiple aspects of MTOC positioning in the one cell stage C. elegans embryo. In conjunction with our observation of cytoplasmic dynein distribution at the periphery of nuclei, these results lead us to propose a mechanism in which cytoplasmic dynein anchored on the nucleus drives centrosome separation.
Figures
Figure 1
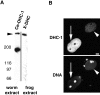
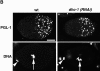
Characterization of anti–DHC-antibodies. (A) Western blot of C. elegans (left lane) or Xenopus (right lane) extracts probed with antibodies raised against a C. elegans DHC-1 peptide (left lane) or a portion of Xenopus DHC (right lane). Both antibodies recognize a similarly migrating very high molecular mass species (arrowhead). In addition, the C. elegans antibodies recognize a species of ∼180 kD. (B) Wild-type (arrow) and dhc-1 (RNAi) (arrowhead) embryos stained with anti–DHC-1 antibodies (top) and counterstained with Hoechst 33258 to reveal DNA (bottom). Anti–DHC-1 staining in this particular dhc-1 (RNAi) embryo is 16.3% of that in the neighboring wild-type embryo. Embryos were also stained with antitubulin antibodies to unambiguously identify dhc-1 (RNAi) embryos (not shown). (C) Comparison of anti–DHC-1 reactivity in wild-type and dhc-1 (RNAi) embryos. On average, 12% of the signal detected in wild-type remains in dhc-1 (RNAi) embryos. Bar, 10 μm.
Figure 1
Characterization of anti–DHC-antibodies. (A) Western blot of C. elegans (left lane) or Xenopus (right lane) extracts probed with antibodies raised against a C. elegans DHC-1 peptide (left lane) or a portion of Xenopus DHC (right lane). Both antibodies recognize a similarly migrating very high molecular mass species (arrowhead). In addition, the C. elegans antibodies recognize a species of ∼180 kD. (B) Wild-type (arrow) and dhc-1 (RNAi) (arrowhead) embryos stained with anti–DHC-1 antibodies (top) and counterstained with Hoechst 33258 to reveal DNA (bottom). Anti–DHC-1 staining in this particular dhc-1 (RNAi) embryo is 16.3% of that in the neighboring wild-type embryo. Embryos were also stained with antitubulin antibodies to unambiguously identify dhc-1 (RNAi) embryos (not shown). (C) Comparison of anti–DHC-1 reactivity in wild-type and dhc-1 (RNAi) embryos. On average, 12% of the signal detected in wild-type remains in dhc-1 (RNAi) embryos. Bar, 10 μm.
Figure 2
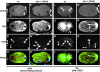
Distribution of cytoplasmic dynein in early wild-type embryos. Embryos stained with anti–DHC-1 and antitubulin (TUB; high magnification images only) antibodies and counterstained with Hoechst 33258 to reveal DNA. A, B, O, and P are at the same magnification, as are C–N. Merged images, DHC-1, red; TUB, green; and DNA, blue. (A and B) One cell stage embryo during pronuclear migration. DHC-1 is distributed throughout the cytoplasm in a punctate manner, and is enriched at the periphery of the male (A and B, arrowhead) and female (A and B, arrow) pronuclei. DHC-1 is also slightly enriched at the cortex of one cell stage embryos, but this is not rendered in this particular focal plane. Small arrowhead in B points to out-of-focus polar body DNA. (C–F) Prometaphase, one cell stage embryo. DHC-1 is enriched on both sides (C and F, arrows) of congressing chromosomes (D and F, arrowhead). (G–J) Metaphase, one cell stage embryo. DHC-1 is enriched on the spindle on both sides of the metaphase plate. (K–N) Early anaphase, P1 blastomere of two cell stage embryo. DHC-1 is enriched on the spindle between the chromosomes (L and N, arrowheads) and the spindle poles, as well as centrally (K and N, arrow) between the two sets of chromosomes. (O and P) Two cell stage embryo, P1 blastomere (right) is in late anaphase, AB blastomere (left) in late telophase. In P1, DHC-1 is enriched on the spindle (O, arrow) between the chromosomes and the spindle poles and in the central spindle. In AB, DHC-1 is enriched at the periphery of reforming nuclei (O, black arrowhead) as well as in an area above the spindle poles. In addition, DHC-1 is localized throughout the cortex between the AB and P1 blastomeres (O, arrowheads). Small arrowhead in P points to polar body DNA. Bars, 10 μm.
Figure 3
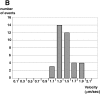
Characterization of rapid minus end–directed movements of yolk granules in wild type. (A) One cell stage embryo in anaphase; high magnification images show an area just anterior of the anterior aster during a 2-s sequence from a time-lapse DIC microscopy recording. Time is indicated in seconds at the right of the frames, which are 10 μm across. One yolk granule (arrows) moves towards the center of the aster (black arrowheads) at an average velocity of 1.40 μm/sec. White arrowheads point to a neighboring yolk granule that remains immobile during the sequence. (B) Histogram of velocities of minus end–directed movement of yolk granules in wild type. Number of motility events per velocity class is shown. Velocity class 1.1 encompasses values from 1.0 to 1.19, velocity class 1.3 values from 1.2 to 1.39 and so forth. On average, motility events (n = 37) lasted 2.7 s (SD 0.85), covered 3.91 μm (SD 1.47), and had a peak velocity of 1.44 μm/s (SD 0.23).
Figure 3
Characterization of rapid minus end–directed movements of yolk granules in wild type. (A) One cell stage embryo in anaphase; high magnification images show an area just anterior of the anterior aster during a 2-s sequence from a time-lapse DIC microscopy recording. Time is indicated in seconds at the right of the frames, which are 10 μm across. One yolk granule (arrows) moves towards the center of the aster (black arrowheads) at an average velocity of 1.40 μm/sec. White arrowheads point to a neighboring yolk granule that remains immobile during the sequence. (B) Histogram of velocities of minus end–directed movement of yolk granules in wild type. Number of motility events per velocity class is shown. Velocity class 1.1 encompasses values from 1.0 to 1.19, velocity class 1.3 values from 1.2 to 1.39 and so forth. On average, motility events (n = 37) lasted 2.7 s (SD 0.85), covered 3.91 μm (SD 1.47), and had a peak velocity of 1.44 μm/s (SD 0.23).
Figure 4
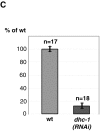
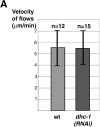
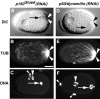
Cytoplasmic flows are not affected in dhc-1 (RNAi) embryos. (A) The average peak velocity of posteriorly directed flow of yolk granules during the pseudocleavage stage is indistinguishable in wild type (5.55 μm/min; n = 12 granules in 5 embryos; SD 1.63) and dhc-1 (RNAi) embryos (5.51 μm/min; n = 15 granules in 5 embryos; SD 1.50). These average velocities are slightly higher than those reported previously in wild type (4.4 μm/min; Hird and White 1993). (B) Wild-type and dhc-1 (RNAi) embryos stained with anti–PGL-1 antibodies to visualize P granules and counterstained with Hoechst 33258 to reveal DNA. All images are at the same magnification. In both wild-type and dhc-1 (RNAi) one cell stage embryos, PGL-1 is segregated to the posterior. Arrowheads point to anteriorly located polar bodies. The wild-type embryo is in prometaphase (arrow points to chromosomes lining up on the metaphase plate), the dhc-1 (RNAi) embryo later in mitosis (arrow points to chromosomes). Embryos were simultaneously stained with antitubulin antibodies (not shown), which revealed the position of centrosomes and unambiguously identified polarity in dhc-1 (RNAi) embryos (Fig. 6). Bar, 10 μm.
Figure 4
Cytoplasmic flows are not affected in dhc-1 (RNAi) embryos. (A) The average peak velocity of posteriorly directed flow of yolk granules during the pseudocleavage stage is indistinguishable in wild type (5.55 μm/min; n = 12 granules in 5 embryos; SD 1.63) and dhc-1 (RNAi) embryos (5.51 μm/min; n = 15 granules in 5 embryos; SD 1.50). These average velocities are slightly higher than those reported previously in wild type (4.4 μm/min; Hird and White 1993). (B) Wild-type and dhc-1 (RNAi) embryos stained with anti–PGL-1 antibodies to visualize P granules and counterstained with Hoechst 33258 to reveal DNA. All images are at the same magnification. In both wild-type and dhc-1 (RNAi) one cell stage embryos, PGL-1 is segregated to the posterior. Arrowheads point to anteriorly located polar bodies. The wild-type embryo is in prometaphase (arrow points to chromosomes lining up on the metaphase plate), the dhc-1 (RNAi) embryo later in mitosis (arrow points to chromosomes). Embryos were simultaneously stained with antitubulin antibodies (not shown), which revealed the position of centrosomes and unambiguously identified polarity in dhc-1 (RNAi) embryos (Fig. 6). Bar, 10 μm.
Figure 5
Failure of pronuclear migration in dhc-1 (RNAi) embryos. Time-lapse DIC microscopy recordings of wild-type (A–D) and dhc-1 (RNAi) embryos (E–H). Time elapsed since the beginning of the sequence is displayed in minutes and seconds in each image. All images are at the same magnification. (A and E) In both wild-type and dhc-1 (RNAi) embryo, the male pronucleus is apposed to the posterior cortex (A and E, rightmost arrow). In wild-type, there is a single female pronucleus located slightly off the anterior cortex (A, leftmost arrow). In contrast, there are five female pronuclei in the dhc-1 (RNAi) embryo (E, arrows towards the left point at three that are visible in this focal plane). Note the pseudocleavage furrow in the middle of both wild-type and dhc-1 (RNAi) embryos. Female pronuclei in some dhc-1 (RNAi) embryos were located towards the middle of the embryo (not shown). (B and F) In wild type, after migration of both male and female pronuclei, the pronuclei have met and move along with the centrosome pair (B, arrowheads) towards the center while undergoing a 90° rotation. In contrast, neither male nor female pronuclei migrate in the dhc-1 (RNAi) embryo. (C and G) In wild type, the spindle sets up in the cell center and along the longitudinal axis (C, arrowheads point to spindle poles). In the dhc-1 (RNAi) embryo, no bipolar structure is visible after nuclear envelope breakdown. However, an area devoid of yolk granules extends towards the anterior of the embryo (arrow in G points to anterior of this area). The asters appear to be at the very posterior of the embryo (G, arrowheads). Note that the membranes of the female pronuclei are still intact after the male pronuclear membrane broke down. (D and H) In wild type, the first cleavage generates two unequally sized daughters, each with a centrally located nucleus (D, arrows). In contrast, no proper cell division occurs in the dhc-1 (RNAi) embryo. While some furrowing activity does take place, this is usually restricted to the anterior and does not result in productive cleavage. Numerous small nuclei reform, presumably around nonsegregated chromosomes, as the cell returns into interphase (H, arrows). Bar, 10 μm.
Figure 6
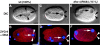
Failure of centrosome separation in dhc-1 (RNAi) embryos. Embryos stained with anti–ZYG-9 and antitubulin (TUB) antibodies and counterstained with Hoechst 33258 to reveal DNA. Images in first two columns: early, before nuclear envelope breakdown (NEB); and prophase. Images in last two columns: late, after NEB. Merged images: ZYG-9, red; TUB, green; and DNA, blue. All images are at the same magnification. (A–D) Wild type, prophase. (A) ZYG-9 labels the two centrosomes (arrowheads), which have separated from one another while remaining associated with the male pronucleus; ZYG-9 is also present in the cytoplasm and polar bodies (small arrowheads). (B) Astral microtubules emanate from the centrosomes; the mesh of cortical microtubules is also visible. (C) DNA of both male (arrow) and female (out of focus, arrowhead) pronuclei is condensing; small arrowheads point to polar body material. (E–F) dhc-1 (RNAi), prophase. (E) Daughter centrosomes (arrowheads) fail to separate from one another and are posterior of the male pronucleus. (F) Some astral microtubules are fairly long (arrow). (G) DNA of both the male (arrow) and the three female (arrowheads) pronuclei is condensing. (I–L) Wild type, anaphase. (I) The two spindle poles (arrowheads) have moved away from each other during anaphase B. (J) Numerous and long astral microtubules extend from the spindle poles towards the anterior and posterior cortices; spindle microtubules extend centrally. (K) The two sets of chromosomes segregate towards the spindle poles; small arrowhead points to polar body material. (M–P) dhc-1 (RNAi); after NEB. (M) Centrosomes (arrowheads) are still in close proximity of one another. (N) No bipolar spindle is assembled; astral microtubules seem to grow preferentially towards chromosomes or be stabilized in their vicinity (arrow); such microtubules are directed towards chromosomes coming presumably from the male pronucleus in most dhc-1 (RNAi) embryos. (O) Condensed chromosomes coming most likely from the male pronucleus (arrow) and the female pronucleus (arrowhead) are visible. Small arrowhead points to laterally positioned polar body material. In 1/46 dhc-1 (RNAi) embryo after NEB, the two centrosomes were separated from one another; a bipolar spindle was not apparent in this case either. Bar, 10 μm.
Figure 7
Failure of pronuclear migration and MTOC separation in p150Glued (RNAi) and p50/dynamitin (RNAi) embryos. (A and D) Single images from time-lapse DIC microscopy recordings of p150Glued (RNAi) (A) and p50/dynamitin (RNAi) (D) embryos. (B, C, E, and F) p150Glued (RNAi) (B and C) and p50/dynamitin (RNAi) embryos (E and F) stained with antitubulin antibodies and counterstained with Hoechst 33258 to reveal DNA. A and D are at the same magnification, as are B, C, E, and F. (A and D) In both p150Glued (RNAi) and p50/dynamitin (RNAi) embryos, no bipolar spindle is visible after NEB. However, an area devoid of yolk granules extends towards the anterior of the embryo (arrows point to anterior of this area). The asters appear to be at the very posterior of the embryos (arrowheads). Note that the membranes of the female pronuclei are still intact after that of the male pronuclei broke down. (B, C, E, and F) In both p150Glued (RNAi) and p50/dynamitin (RNAi) embryos, the two MTOCs are in close proximity at the very posterior of the embryo (B and E, arrowheads). p150Glued (RNAi) embryo is just after breakdown of the male pronucleus (C, arrow); arrowhead in C points to condensed chromosomes from the female pronucleus. p50/dynamitin (RNAi) embryo is towards the end of prophase, before breakdown of the male pronucleus. At these stages in wild type, centrosomes are well separated (Fig. 6). Small arrowheads in C and F point to polar body material. Bars, 10 μm.
Figure 8
Centrosomes and male pronucleus are not tightly associated in some dhc-1 (RNAi) embryos. (A, C, and E) Time-lapse DIC microscopy of wild-type (A) and dhc-1 (RNAi) embryos (C and E). A is from the wild-type embryo shown in Fig. 5. (B, D, and F) Wild-type (B) and dhc-1 (RNAi) embryos (D and F) stained with anti–ZYG-9 antibodies and counterstained with Hoechst 33258 to reveal DNA. B and D are from embryos shown in Fig. 6. Merged images, ZYG-9, red; DNA, blue. A, C, and E are at the same magnification, as are B, D, and F. (A and B) In wild type, centrosomes are initially associated with the male pronucleus, and with both male and female pronuclei after pronuclear meeting. (A) The center of the asters is visible by DIC microscopy as areas excluding yolk granules (arrowheads); arrows point to associated pronuclei. (B) Anti–ZYG-9 labeling demonstrates that the two centrosomes (arrowheads) are associated with the male pronucleus (arrow). (C and D) In 85% of dhc-1 (RNAi) embryos, unseparated centrosomes are in the immediate vicinity of the male pronucleus. (C) An area lacking yolk granules and corresponding to the center of the asters (arrowheads) is visible just posterior of the male pronucleus (arrow). (D) Anti–ZYG-9 labeling demonstrates that unseparated centrosomes (arrowheads) are located in the immediate vicinity and posterior of the male pronucleus (arrow). (E and F) In 15% of dhc-1 (RNAi) embryos, unseparated centrosomes are located 3–11-μm away from the male pronucleus. (E) The area lacking yolk granules and corresponding to the center of the asters (arrowheads) is located 6.5 μm away from the male pronucleus (arrow) in this particular embryo. (F) Anti–ZYG-9 labeling reveals that unseparated centrosomes (arrowheads) are located 7.94-μm away from the male pronucleus (arrow) in this particular embryo. In 1 of 7 embryos where association was compromised, the two centrosomes were separated from one another, and only one of them was not associated with the male pronucleus. Although the embryo shown in F is too early in the cell cycle to be unambiguously scoreable for the occurrence of centrosome duplication, there were two centrosomes in 91/91 one cell stage dhc-1 (RNAi) embryos examined during prophase or after NEB. Bars, 10 μm.
Figure 9
Spindle orientation defect in dhc-1 (ssRNAi) embryos. Time-lapse DIC microscopy recordings of wild-type (A–C) and dhc-1 (ssRNAi) (D–F) embryos. In each image, time elapsed since the beginning of the sequence is displayed in minutes and seconds and arrowheads point to center of asters and spindle poles. All images are at the same magnification. (A and D) In both wild-type and dhc-1 (ssRNAi) embryos, pronuclei meet at ∼70% egg length. (B and E) In wild type, the centrosome pair and associated pronuclei move towards the center while undergoing a 90° rotation. This does not happen in the dhc-1 (ssRNAi) embryo. As a result, while the spindle sets up in the cell center and along the longitudinal axis in wild-type, it does so in the posterior half and perpendicular to the longitudinal axis in the dhc-1 (ssRNAi) embryo. (C and F) Both wild-type and dhc-1 (ssRNAi) embryos divide asymmetrically into a larger anterior blastomere and a smaller posterior one. In the dhc-1 (ssRNAi) embryo, this occurs after rescue of the spindle orientation onto the longitudinal axis during anaphase, possibly because of the physical constraints of the eggshell. Note that the cleavage furrow ingresses sooner on one side of the dhc-1 (ssRNAi) embryo (bottom side), because the spindle was closer to that side during rescue of spindle orientation. In some dhc-1 (ssRNAi) embryos, >1 nucleus reformed in each daughter blastomere, indicative of defects in chromosome segregation. Bar, 10 μm.
Figure 10
Possible model of dynein-dependent separation of centrosomes. View is on posterior of one cell stage embryo. Male pronucleus: large light disk. Centrosomes: small black disks. Shown also are astral microtubules (black lines) and two-headed cytoplasmic dynein; cytoplasmic dynein molecules that interact with astral microtubules are shown in dark shading, others in light shading. Cytoplasmic dynein is evenly distributed on the male pronucleus. When astral microtubules encounter such anchored motors, their minus end is pulled towards cytoplasmic dynein, along with the centrosome. Longer astral microtubules encounter more motors and, thus, experience a stronger pulling force than shorter ones. Because astral microtubules growing towards the anterior are longer to start with, dynein-dependent forces displace centrosomes towards the anterior initially. When astral microtubules growing in all directions along the nuclear envelope are of equal size, centrosome movement stops. In this model, cytoplasmic dynein also serves to couple male pronucleus and centrosomes. See text for additional information.
Similar articles
- lis-1 is required for dynein-dependent cell division processes in C. elegans embryos.
Cockell MM, Baumer K, Gönczy P. Cockell MM, et al. J Cell Sci. 2004 Sep 1;117(Pt 19):4571-82. doi: 10.1242/jcs.01344. J Cell Sci. 2004. PMID: 15331665 - Functional analysis of cytoplasmic dynein heavy chain in Caenorhabditis elegans with fast-acting temperature-sensitive mutations.
Schmidt DJ, Rose DJ, Saxton WM, Strome S. Schmidt DJ, et al. Mol Biol Cell. 2005 Mar;16(3):1200-12. doi: 10.1091/mbc.e04-06-0523. Epub 2004 Dec 22. Mol Biol Cell. 2005. PMID: 15616192 Free PMC article. - Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium.
Ma S, Triviños-Lagos L, Gräf R, Chisholm RL. Ma S, et al. J Cell Biol. 1999 Dec 13;147(6):1261-74. doi: 10.1083/jcb.147.6.1261. J Cell Biol. 1999. PMID: 10601339 Free PMC article. - [Dynein and dynactin as organizers of the system of cell microtubules].
Burakov AV, Nadezhdina ES. Burakov AV, et al. Ontogenez. 2006 Sep-Oct;37(5):323-39. Ontogenez. 2006. PMID: 17066975 Review. Russian. - Cytoplasmic dynein and dynactin in cell division and intracellular transport.
Karki S, Holzbaur EL. Karki S, et al. Curr Opin Cell Biol. 1999 Feb;11(1):45-53. doi: 10.1016/s0955-0674(99)80006-4. Curr Opin Cell Biol. 1999. PMID: 10047518 Review.
Cited by
- Stronger net posterior cortical forces and asymmetric microtubule arrays produce simultaneous centration and rotation of the pronuclear complex in the early Caenorhabditis elegans embryo.
Coffman VC, McDermott MB, Shtylla B, Dawes AT. Coffman VC, et al. Mol Biol Cell. 2016 Nov 7;27(22):3550-3562. doi: 10.1091/mbc.E16-06-0430. Epub 2016 Oct 12. Mol Biol Cell. 2016. PMID: 27733624 Free PMC article. - Specific kinesin and dynein molecules participate in the unconventional protein secretion of transmembrane proteins.
Eun SH, Noh SH, Lee MG. Eun SH, et al. Korean J Physiol Pharmacol. 2024 Sep 1;28(5):435-447. doi: 10.4196/kjpp.2024.28.5.435. Korean J Physiol Pharmacol. 2024. PMID: 39198224 Free PMC article. - Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration.
Tanaka T, Serneo FF, Higgins C, Gambello MJ, Wynshaw-Boris A, Gleeson JG. Tanaka T, et al. J Cell Biol. 2004 Jun 7;165(5):709-21. doi: 10.1083/jcb.200309025. Epub 2004 Jun 1. J Cell Biol. 2004. PMID: 15173193 Free PMC article. - Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes.
Young A, Dictenberg JB, Purohit A, Tuft R, Doxsey SJ. Young A, et al. Mol Biol Cell. 2000 Jun;11(6):2047-56. doi: 10.1091/mbc.11.6.2047. Mol Biol Cell. 2000. PMID: 10848628 Free PMC article. - The roles of microtubule-based motor proteins in mitosis: comprehensive RNAi analysis in the Drosophila S2 cell line.
Goshima G, Vale RD. Goshima G, et al. J Cell Biol. 2003 Sep 15;162(6):1003-16. doi: 10.1083/jcb.200303022. J Cell Biol. 2003. PMID: 12975346 Free PMC article.
References
- Albertson D. Formation of the first cleavage spindle in nematode embryos. Dev. Biol. 1984;101:61–72. - PubMed
- Albertson D.G., Thomson J.N. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans . Chromosome Res. 1993;1:15–26. - PubMed
- Allan V. Organelle movement. Dynactinportrait of a dynein regulator. Curr. Biol. 1994;4:1000–1002. - PubMed
- Bajer A.S., Mole-Bajer J. Asters, poles, and transport properties within spindlelike microtubule arrays. Cold Spring Harb. Symp. Quant. Biol. 1982;1:263–283. - PubMed
- Boleti H., Karsenti E., Vernos I. XKLP2, a novel Xenopus centrosomal kinesin-like protein required for centrosome separation during mitosis. Cell. 1996;84:49–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials