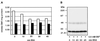Exogenous administration of gangliosides displaces GPI-anchored proteins from lipid microdomains in living cells - PubMed (original) (raw)
Exogenous administration of gangliosides displaces GPI-anchored proteins from lipid microdomains in living cells
M Simons et al. Mol Biol Cell. 1999 Oct.
Free PMC article
Abstract
Exogenous application of gangliosides to cells affects many cellular functions. We asked whether these effects could be attributed to the influence of gangliosides on the properties of sphingolipid-cholesterol microdomains on the plasma membrane, also termed rafts. The latter are envisaged as lateral assemblies of sphingolipids (including gangliosides), cholesterol, and a specific set of proteins. Rafts have been implicated in processes such as membrane trafficking, signal transduction, and cell adhesion. Recently, using a chemical cross-linking approach with Madin-Darby canine kidney (MDCK) cells permanently expressing a GPI-anchored form of growth hormone decay accelerating factor (GH-DAF) as a model system, we could show that GPI-anchored proteins are clustered in rafts in living cells. Moreover, this clustering was dependent on the level of cholesterol in the cell. Here we show that incubation of MDCK cells with gangliosides abolished subsequent chemical cross-linking of GH-DAF. Furthermore, insertion of gangliosides into the plasma membrane of MDCK GH-DAF cells renders GH-DAF soluble when subjected to extraction with Triton X-114 at 4 degrees C. Our data suggest that exogenous application of gangliosides displaces GPI-anchored proteins from sphingolipid-cholesterol microdomains in living cells.
Figures
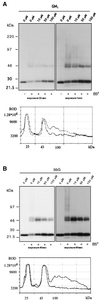
Figure 1
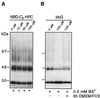
Gangliosides inhibit cross-linking of GH-DAF in MDCK cells. MDCK GH-DAF cells were incubated with 0, 10, 50, and 100 μM GM1 (A) or with 10, 50, and 100 μM bbG (B) for 1 h at 37°C. Cells were subsequently subjected to cross-linking with BS3. Proteins were resolved on 5–15% SDS-PAGE and after Western blotting were detected with anti-GH antibody followed by ECL. Western blots were scanned, and the intensity of immunoreactive signal is given as relative optical density (ROD) for 0 μM GM1 (A, bottom curve), 0 μM bbG (B, bottom curve), 100 μM GM1 (A, top curve), and 100 μM bbG (B, top curve).
Figure 2
Gangliosides inhibit cross-linking of FR-GPI in CHO cells. CHO FR-GPI cells were incubated with 100 μM bbG or with 100 μM GM1 for 1 h at 37°C. Cells were subsequently subjected to cross-linking with BS3. Proteins were resolved on 5–15% SDS-PAGE and after Western blotting were detected with antifolate receptor antibody followed by ECL.
Figure 3
Incorporation of GM1 into the plasma membrane of MDCK GH-DAF cells. (A) MDCK GH-DAF cells were incubated with 1 μCi/ml tritiated GM1 (final ganglioside concentration 100 μM) for 1 h at 37°C and subsequently washed with BSA for 0, 10, 20, 30, or 45 min (open bars). The additive effect of trypsin treatment (0.1% trypsin for 5 min at 37°C) after washing with BSA is shown as solid bars for each time point. (B) Cross-linking was performed after incubating GM1-loaded cells with BSA for 0, 10, 20, 30, or 45 min. SDS-PAGE, Western blotting, and detection were as described in Figure 1.
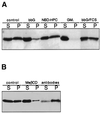
Figure 4
Gangliosides increase the detergent solubility of GH-DAF. (A) MDCK GH-DAF cells were loaded with 100 μM bbG for 1 h at 37°C, with 100 μM NBD-C6-HPC for 1 h at 8°C, with 100 μM GM1 for 1 h at 37°C, or with 100 μM bbG for 1 h followed by a 6-h incubation period with serum containing DMEM (A) or treated with 10 mM methyl-β-cyclodextrin for 1 h at 37°C or incubated with anti-GH for 1 h at 12°C followed by incubation with secondary antibody (B). Cells were extracted with Triton X-114 for 30 min at 4°C and subjected to centrifugation, and GH-DAF in the soluble (S) and insoluble fraction (I) was detected as described in MATERIALS AND METHODS.
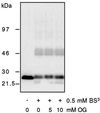
Figure 5
The effect of octylglucoside on rafts is different from that of gangliosides. MDCK GH-DAF cells were incubated for 1 h with 5 and 10 mM octylglucoside and subjected to cross-linking with BS3. A slight decrease of cross-linking was observed only with 10 mM OG. For all concentrations tested, membranes remained intact (see RESULTS).
Figure 6
The inhibition of cross-linking of GH-DAF is a specific property of gangliosides. (A) MDCK GH-DAF cells were incubated with 0, 10, 50, or 100 μM NBD-C6-HPC for 30 min at 8°C before being subjected to cross-linking with BS3. SDS-PAGE, Western blotting, and detection were as described in Figure 1. (B) MDCK GH-DAF cells were incubated with 0 or 100 μM bbG for 1 h followed by a 6-h incubation with serum-containing medium as indicated in the figure. Cells were subjected to cross-linking followed by SDS-PAGE, Western blotting, and detection as detailed in Figure 1.
Figure 7
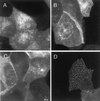
Ganglioside treatment does not change the immunofluorescence pattern of GH-DAF. MDCK GH-DAF cells were incubated without any additions (A and D), with 100 μM bbG (B), and with 10 mM CD (C) for 1 h at 37°C, respectively. Cells were fixed and labeled with anti-GH antibody followed by Cy3-conjugated anti-sheep IgG (A–C) or subjected to antibody-induced cross-linking before fixation (D) as described in MATERIALS AND METHODS.
Figure 8
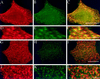
Inhibition of copatching of influenza HA and GH-DAF by gangliosides. MDCK GH-DAF cells were infected with influenza HA virus and then incubated with DMEM (A–F) or 100 μM GM1 in DMEM (G–L) for 1 h at 37°C. After subsequent treatment with a mixture of monoclonal anti-HA and sheep polyclonal anti-GH antibodies at 4°C, patching was detected using Cy-3-anti-sheep–labeled (red) and FITC-anti-mouse–labeled (green) secondary antibodies. Panels in the left column show distribution of GH, panels in the middle column show distribution of HA, and panels in the right column show the merge of both signals. D–F display a detail of A–C correspondingly, whereas J–L display that of G–I. Bars: F, L, 2 μm; C, I, 8 μm.
Similar articles
- Effect of gangliosides on the distribution of a glycosylphosphatidylinositol-anchored protein in plasma membrane from Chinese hamster ovary-K1 cells.
Crespo PM, Zurita AR, Daniotti JL. Crespo PM, et al. J Biol Chem. 2002 Nov 22;277(47):44731-9. doi: 10.1074/jbc.M204604200. Epub 2002 Sep 16. J Biol Chem. 2002. PMID: 12237294 - Microdomains of GPI-anchored proteins in living cells revealed by crosslinking.
Friedrichson T, Kurzchalia TV. Friedrichson T, et al. Nature. 1998 Aug 20;394(6695):802-5. doi: 10.1038/29570. Nature. 1998. PMID: 9723622 - N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells.
Benting JH, Rietveld AG, Simons K. Benting JH, et al. J Cell Biol. 1999 Jul 26;146(2):313-20. doi: 10.1083/jcb.146.2.313. J Cell Biol. 1999. PMID: 10427087 Free PMC article. - Biosynthesis, remodelling and functions of mammalian GPI-anchored proteins: recent progress.
Kinoshita T, Fujita M, Maeda Y. Kinoshita T, et al. J Biochem. 2008 Sep;144(3):287-94. doi: 10.1093/jb/mvn090. Epub 2008 Jul 17. J Biochem. 2008. PMID: 18635593 Review. - GPI-anchored proteins and lipid rafts.
Sangiorgio V, Pitto M, Palestini P, Masserini M. Sangiorgio V, et al. Ital J Biochem. 2004 Jul;53(2):98-111. Ital J Biochem. 2004. PMID: 15646015 Review.
Cited by
- Microvesicles/exosomes as potential novel biomarkers of metabolic diseases.
Müller G. Müller G. Diabetes Metab Syndr Obes. 2012;5:247-82. doi: 10.2147/DMSO.S32923. Epub 2012 Aug 7. Diabetes Metab Syndr Obes. 2012. PMID: 22924003 Free PMC article. - Lipid rafts make for slippery platforms.
Lai EC. Lai EC. J Cell Biol. 2003 Aug 4;162(3):365-70. doi: 10.1083/jcb.200307087. Epub 2003 Jul 28. J Cell Biol. 2003. PMID: 12885764 Free PMC article. Review. - Functional roles of glycosphingolipids in signal transduction via lipid rafts.
Kasahara K, Sanai Y. Kasahara K, et al. Glycoconj J. 2000 Mar-Apr;17(3 -4):153-62. doi: 10.1023/a:1026576804247. Glycoconj J. 2000. PMID: 11201786 Review. - GD1a Overcomes Inhibition of Myelination by Fibronectin via Activation of Protein Kinase A: Implications for Multiple Sclerosis.
Qin J, Sikkema AH, van der Bij K, de Jonge JC, Klappe K, Nies V, Jonker JW, Kok JW, Hoekstra D, Baron W. Qin J, et al. J Neurosci. 2017 Oct 11;37(41):9925-9938. doi: 10.1523/JNEUROSCI.0103-17.2017. Epub 2017 Sep 12. J Neurosci. 2017. PMID: 28899916 Free PMC article. - Role of lipid rafts and GM1 in the segregation and processing of prion protein.
Botto L, Cunati D, Coco S, Sesana S, Bulbarelli A, Biasini E, Colombo L, Negro A, Chiesa R, Masserini M, Palestini P. Botto L, et al. PLoS One. 2014 May 23;9(5):e98344. doi: 10.1371/journal.pone.0098344. eCollection 2014. PLoS One. 2014. PMID: 24859148 Free PMC article.
References
- Bremer EG, Hakomori S, Bowen-Pope DF, Raines EW, Ross R. Ganglioside-mediated modulation of cell growth, growth factor binding, and receptor phosphorylation. J Biol Chem. 1984;259:6818–6825. - PubMed
- Bremer EG, Schlessinger J, Hakomori S. Ganglioside-mediated modulation of cell growth: specific effects of GM3 on tyrosine phosphorylation of the epidermal growth factor receptor. J Biol Chem. 1986;261:2434–2440. - PubMed
- Brown D. The tyrosine kinase connection: how GPI-anchored proteins activate T cells. Curr Opin Immunol. 1993;5:349–354. - PubMed
- Brown DA. Interactions between GPI-anchored proteins and membrane lipids. Trends Cell Biol. 1992;2:338–343. - PubMed
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous