Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers - PubMed (original) (raw)
Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers
N Sévenet et al. Am J Hum Genet. 1999 Nov.
Abstract
Biallelic, truncating mutations of the hSNF5/INI1 gene have recently been documented in malignant rhabdoid tumor (MRT), one of the most aggressive human cancers. This finding suggests that hSNF5/INI1 is a new tumor-suppressor gene for which germline mutations might predispose to cancer. We now report the presence of loss-of-function mutations of this gene in the constitutional DNA from affected members but not from healthy relatives in cancer-prone families. Furthermore, a constitutional mutation is documented in a patient with two successive primary cancers. In agreement with the two-hit model, the wild-type hSNF5/INI1 allele is deleted in the tumor DNA from mutation carriers. In all tested cases, DNA from parents demonstrated normal hSNF5/INI1 sequences, therefore indicating the de novo occurrence of the mutation, which was shown to involve the maternal allele in one case and the paternal allele in two other cases. These data indicate that constitutional mutation of the hSNF5/INI1 gene defines a new hereditary syndrome predisposing to renal or extrarenal MRT and to a variety of tumors of the CNS, including choroid plexus carcinoma, medulloblastoma, and central primitive neuroectodermal tumor. This condition, which we propose to term "rhabdoid predisposition syndrome," may account for previous observations of familial and multifocal cases of the aforementioned tumor types. It could also provide the molecular basis for cases of Li-Fraumeni syndrome without p53 germline mutations.
Figures
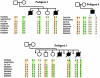
Figure 1
Schematic representation of four pedigrees with mutations of hSNF5/INI1. The results of the molecular analysis are shown below each family member. Normal and italic characters indicate the results of the analysis of constitutional DNA and tumor DNA, respectively. N = normal sequence; a virgule (/) indicates that DNA was not available for analysis. Mb = medulloblastoma; other abbreviations are as defined in the text. The age (in years) of the healthy sibs is indicated. In pedigree 4, the results of the DNA analysis of the two tumors (T1 and T2) are given.
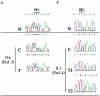
Figure 2
Loss of the wild-type hSNF5/INI1 allele in tumors. The results for the male child in pedigree 3 who had ATTR (II.6) and for the patient in pedigree 4 who had bifocal tumors (II.1) are depicted. The normal sequences (N) are shown at the top; the constitutional sequences (C) that demonstrate heterozygosity between wild-type and mutated alleles are shown below them. a, Case II.6 from pedigree 3. The sequence of the ATTR DNA (T) shows the mutated 591delG allele with complete disappearance of the wild-type allele. b, Case II.1 from pedigree 4. DNA from the two successive tumors (T1 [renal MRT] and T2 [central PNET]) demonstrates the presence of the 601T mutated allele and the loss of the wild-type 601C allele. Therefore, in these three tumors, no functional allele of hSNF5/INI1 was retained.
Figure 3
Parental origin of the mutated allele. Haplotypes around the hSNF5/INI1 gene on chromosome 22 are displayed for pedigrees 2–4. Paternal and maternal haplotypes are indicated on the left and right, respectively. The seven microsatellite markers shown on the left are included within a 3-cM region (Dib et al. 1996). “C” and “T” denote constitutional DNA and tumor DNA, respectively. The point mutations of hSNF5/INI1 are depicted by red crosses. In pedigree 3, the mutation is carried by the maternal allele, whereas in pedigrees 2 and 4 it involves the paternal allele.
Similar articles
- Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype-phenotype correlations.
Sévenet N, Lellouch-Tubiana A, Schofield D, Hoang-Xuan K, Gessler M, Birnbaum D, Jeanpierre C, Jouvet A, Delattre O. Sévenet N, et al. Hum Mol Genet. 1999 Dec;8(13):2359-68. doi: 10.1093/hmg/8.13.2359. Hum Mol Genet. 1999. PMID: 10556283 - hSNF5/INI1-deficient tumours and rhabdoid tumours are convergent but not fully overlapping entities.
Bourdeaut F, Fréneaux P, Thuille B, Lellouch-Tubiana A, Nicolas A, Couturier J, Pierron G, Sainte-Rose C, Bergeron C, Bouvier R, Rialland X, Laurence V, Michon J, Sastre-Garau X, Delattre O. Bourdeaut F, et al. J Pathol. 2007 Feb;211(3):323-30. doi: 10.1002/path.2103. J Pathol. 2007. PMID: 17152049 - Familial posterior fossa brain tumors of infancy secondary to germline mutation of the hSNF5 gene.
Taylor MD, Gokgoz N, Andrulis IL, Mainprize TG, Drake JM, Rutka JT. Taylor MD, et al. Am J Hum Genet. 2000 Apr;66(4):1403-6. doi: 10.1086/302833. Epub 2000 Mar 14. Am J Hum Genet. 2000. PMID: 10739763 Free PMC article. - Integrase interactor 1 in health and disease.
Das S. Das S. Curr Protein Pept Sci. 2015;16(6):478-90. doi: 10.2174/1389203716666150316115156. Curr Protein Pept Sci. 2015. PMID: 25772157 Review. - Atypical teratoid/rhabdoid tumors and choroid plexus tumors: when genetics "surprise" pathology.
Gessi M, Giangaspero F, Pietsch T. Gessi M, et al. Brain Pathol. 2003 Jul;13(3):409-14. doi: 10.1111/j.1750-3639.2003.tb00039.x. Brain Pathol. 2003. PMID: 12946029 Free PMC article. Review.
Cited by
- Clinical protocol phase II study of tumor infiltrating lymphocytes in advanced tumors with alterations in the SWI/SNF complex: the TILTS study.
Martin-Liberal J, Garralda E, García-Donas J, Soto-Castillo JJ, Mussetti A, Codony C, Martin-Lluesma S, Muñoz S, Galvao V, Lostes J, Rotxes M, Prat-Vidal C, Palomero J, Muñoz A, Moreno R, García Del Muro X, Sureda A, Alemany R, Gros A, Piulats JM. Martin-Liberal J, et al. Future Oncol. 2024;20(32):2437-2445. doi: 10.1080/14796694.2024.2385287. Epub 2024 Aug 12. Future Oncol. 2024. PMID: 39129675 Clinical Trial. - Late-onset tumors in rhabdoid tumor predisposition syndrome type-1 (RTPS1) and implications for surveillance.
Nakano Y, Acker M, Druker H, van Engelen K, Meyn MS, Wasserman JD, Venier RE, Goudie C, Stosic A, Huang A, Greer MC, Malkin D, Villani A, Gallinger B. Nakano Y, et al. Eur J Hum Genet. 2024 Nov;32(11):1474-1482. doi: 10.1038/s41431-024-01674-z. Epub 2024 Aug 8. Eur J Hum Genet. 2024. PMID: 39117932 - Postzygotic mosaicism of SMARCB1 variants in patients with rhabdoid tumors: A not-so-rare condition exposing to successive tumors.
Thomson G, Filser M, Guerrini-Rousseau L, Tauziede-Espariat A, Bourneix C, Gauthier-Villars M, Simaga F, Beccaria K, Faure-Conter C, Maureille A, Zattara-Cannoni H, Andre N, Entz-Werle N, Brugieres L, Mansuy L, Denizeau P, Julia S, Ingster O, Lejeune S, Brahimi A, Coupier I, Bonadona V, Delattre O, Masliah-Planchon J, Bourdeaut F. Thomson G, et al. Neuro Oncol. 2024 Nov 4;26(11):2102-2112. doi: 10.1093/neuonc/noae122. Neuro Oncol. 2024. PMID: 39093628 - Chromatin remodellers as therapeutic targets.
Malone HA, Roberts CWM. Malone HA, et al. Nat Rev Drug Discov. 2024 Sep;23(9):661-681. doi: 10.1038/s41573-024-00978-5. Epub 2024 Jul 16. Nat Rev Drug Discov. 2024. PMID: 39014081 Review. - Rhabdoid tumor predisposition syndrome: A historical review of treatments and outcomes for associated pediatric malignancies.
Andres S, Huang K, Shatara M, Abdelbaki MS, Ranalli M, Finlay J, Gupta A. Andres S, et al. Pediatr Blood Cancer. 2024 Jun;71(6):e30979. doi: 10.1002/pbc.30979. Epub 2024 Mar 30. Pediatr Blood Cancer. 2024. PMID: 38553892 Review.
References
Electronic-Database Information
- EMBL database, http://www.ebi.ac.uk/cgi-bin/emblfetch (for sequences of the intron-exon boundaries [Y17118-Y17126])
- Institut Curie, http://www.curie.fr/sr/unites/u509 (for sequences of the primer pairs used for PCR together with the dHPLC conditions)
References
- Beckwith JB, Palmer NF (1978) Histopathology and prognosis of Wilms tumor. Cancer 41:1937–1948 - PubMed
- Biegel JA, Allen CS, Kawasaki K, Shimizu N, Budarf ML, Bell CJ (1996) Narrowing the critical region for a rhabdoid tumor locus in 22q11. Genes Chromosomes Cancer 16:94–105 - PubMed
- Biegel JA, Burk CD, Parmiter AH, Emanuel BS (1992) Molecular analysis of a partial deletion of 22q in a central nervous system rhabdoid tumor. Genes Chromosomes Cancer 5:104–108 - PubMed
- Biegel JA, Zhou JY, Rorke LB, Stenstrom C, Wainwright LM, Fogelgren B (1999) Germ-line and acquired mutations of INI1 in atypical teratoid rhabdoid tumors. Cancer Res 59:74–79 - PubMed
- Bonnin JM, Rubinstein LJ, Palmer NF, Beckwith JB (1984) The association of embryonal tumors originating in the kidney and in the brain: a report of seven cases. Cancer 54:2137–2146 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous