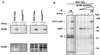Functional independence and interdependence of the Src homology domains of phospholipase C-gamma1 in B-cell receptor signal transduction - PubMed (original) (raw)
Functional independence and interdependence of the Src homology domains of phospholipase C-gamma1 in B-cell receptor signal transduction
K E DeBell et al. Mol Cell Biol. 1999 Nov.
Abstract
B-cell receptor (BCR)-induced activation of phospholipase C-gamma1 (PLCgamma1) and PLCgamma2 is crucial for B-cell function. While several signaling molecules have been implicated in PLCgamma activation, the mechanism coupling PLCgamma to the BCR remains undefined. The role of PLCgamma1 SH2 and SH3 domains at different steps of BCR-induced PLCgamma1 activation was examined by reconstitution in a PLCgamma-negative B-cell line. PLCgamma1 membrane translocation required a functional SH2 N-terminal [SH2(N)] domain, was decreased by mutation of the SH3 domain, but was unaffected by mutation of the SH2(C) domain. Tyrosine phosphorylation did not require the SH2(C) or SH3 domains but depended exclusively on a functional SH2(N) domain, which mediated the association of PLCgamma1 with the adapter protein, BLNK. Forcing PLCgamma1 to the membrane via a myristoylation signal did not bypass the SH2(N) domain requirement for phosphorylation, indicating that the phosphorylation mediated by this domain is not due to membrane anchoring alone. Mutation of the SH2(N) or the SH2(C) domain abrogated BCR-stimulated phosphoinositide hydrolysis and signaling events, while mutation of the SH3 domain partially decreased signaling. PLCgamma1 SH domains, therefore, have interrelated but distinct roles in BCR-induced PLCgamma1 activation.
Figures
FIG. 1
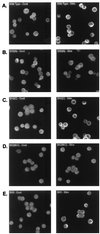
BCR-induced translocation of PLCγ1 to the cell membrane is affected by mutation of the SH2(N) domain or SH3 domain. Stable transfectants, expressing similar levels of PLCγ1-HA by Western blot analysis, were treated with medium (left panels, Cont) or anti-chicken IgM (right panels, Stim) for 1 min, fixed with formaldehyde, permeabilized, reacted with anti-HA Ab, and stained with a secondary FITC-conjugated antiserum as described in Materials and Methods. (A) PLCγ1-HA WT; (B) PLCγ1-HA SH2(N)R586K; (C) PLCγ1-HA SH2(C)R694/6K; (D) PLCγ1-HA SH2(N/C)R586/694/6K; (E) PLCγ1-HA SH3P842L.
FIG. 2
BCR-induced translocation of PLCγ1 to the membrane fraction is affected by mutation of the SH2(N) domain and SH3 domain. (A) Stable transfectants expressing WT PLCγ1-HA were precoated with a murine anti-chicken IgM monoclonal Ab and stimulated for 1 min at 37°C with a rabbit anti-mouse IgG Ab. Medium-treated (uncoated) cells or cells treated only with the rabbit anti-murine IgG serum (Sec Ab) were included as controls. Cytosol and particulate (membrane) fractions were resolved by SDS-PAGE and immunoblotted with anti-HA. (B) Resolubilized membrane fractions from control- or BCR-activated (1 min at 37°C) stable transfectants expressing identical levels of the indicated PLCγ1-HA WT and mutant proteins were resolved by SDS-PAGE and immunoblotted with anti-HA.
FIG. 3
BCR-induced tyrosine phosphorylation of PLCγ1 requires an intact SH2(N) domain. Stable WT or SH mutant PLCγ1-HA transfectants were stimulated with anti-chicken IgM for 1 min at 37°C. Anti-HA immunoprecipitates were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine (upper panels). The same blots were stripped and reprobed with anti-HA (lower panels) for comparison of the relative amounts of PLCγ1-HA expressed. HC, heavy chain; LC, light chain.
FIG. 4
pp80 binds the SH2 domains of PLCγ1 or Grb2. (A) Untransfected P10-14 cells were treated with medium or anti-chicken IgM for 1 min. Lysates were precipitated with GST alone or with the GST-SH2(N) or GST-SH2(C) domain fusion protein of PLCγ1 or a GST-Grb2 fusion protein immobilized onto glutathione-Sepharose beads. Lysates from stable P10-14 transfectants expressing WT PLCγ1-HA were subjected to immunoprecipitation (IP) with anti-HA. Proteins were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine. (B) Lysates from stable P10-14 transfectants expressing WT PLCγ1-HA were immunoprecipitated with anti-HA or were sequentially precipitated with three rounds of GST-Grb2 fusion protein followed by anti-HA immunoprecipitation. Proteins were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine. HC, heavy chain; LC, light chain.
FIG. 5
The PLCγ1 SH2(N) domain-bound pp80 is BLNK. (A) Stable P10-14 transfectants expressing WT PLCγ1-HA or PLCγ1-HA SH2(N)R586K domain mutant were treated for 1 min at 37°C with medium alone or anti-chicken IgM. Lysates were immunoprecipitated with anti-HA and proteins resolved by SDS-PAGE. Blots were probed with antiphosphotyrosine (upper panels) and stripped and reprobed with anti-chicken BLNK (lower panels). (B) Lysates from activated WT PLCγ1-HA transfectants were immunoprecipitated (IP) with anti-HA or were sequentially immunoprecipitated with anti-chicken BLNK followed by anti-HA immunoprecipitation. Samples were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine. HC, heavy chain.
FIG. 6
Membrane targeting of PLCγ1 via myristoylation does not bypass the SH2(N) domain requirement for BCR-induced tyrosine phosphorylation. (A) P10-14 cells were transiently transfected with 20 μg of pCIneo PLCγ1-HA WT or pCIneo PLCγ1-HA SH2(N)R586K containing an amino-terminal Src myr myr sequence or a control sequence (cont). After 24 h, cells were lysed by Dounce homogenization and fractionated into cytosol (Cyto) and membrane (Membr) components. Twenty-five micrograms of protein was resolved by SDS-PAGE and immunoblotted with anti-HA. (B) Densitometric analysis of the blot shown in panel A. (C) Transiently transfected P10-14 cells were treated for 1 min with medium or anti-chicken IgM. Lysates were immunoprecipitated with anti-HA, resolved by SDS-PAGE, and probed with antiphosphotyrosine (upper panel). The same blot was stripped and reprobed with anti-HA (lower panel) for comparison of the relative amounts of PLCγ1-HA expressed. HC, heavy chain; LC, light chain.
FIG. 7
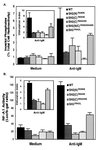
PLCγ1 SH domains are required for BCR-induced phosphoinositide hydrolysis and NF-AT induction. (A) Cells (2 × 106) expressing PLCγ1-HA WT and mutant constructs were labeled with [3H]inositol, washed, and incubated with 5 × 107 3-μm-diameter polystyrene beads precoated with bovine albumin or anti-chicken IgM. Inositol phosphates were allowed to accumulated for 45 min at 37°C in the presence of 5 mM LiCl and separated from free inositol by ion-exchange chromatography. Data are expressed as the percentage of total cell-associated radioactivity (mean ± standard deviation of three separate experiments). The inset represents the data normalized per the resting, unstimulated levels of inositol phosphates (stimulation index). (B) Stable PLCγ1-HA transfectants were transiently transfected with pNF-AT-Luc and pADβ for 24 h. Cells were stimulated for 6 h with plate-immobilized anti-chicken IgM, harvested, lysed, and assayed for luciferase and β-galactosidase activities. Specific NF-AT activity is expressed as the luciferase/β-galactosidase (b-gal) ratio (mean ± standard deviation of three separate experiments). The inset represents the data normalized per the resting, unstimulated NF-AT activity (stimulation index).
FIG. 8
Influence of the SH2 domains on the in vitro enzymatic activity of PLCγ1 from resting and BCR-activated cells. Cells stably expressing PLCγ1-HA WT, the PLCγ1-HA SH2(N)R586K domain mutant, or the PLCγ1-HA SH2(C)R694/6K domain mutant were treated with medium alone or with anti-BCR Ab for 2 min at 37°C. Anti-HA immunoprecipitates from 2.5 × 107 cells were assayed for PLC activity by using a mixed micellar suspension of [3H]PtdInsP2 and Triton X-100 as the substrate. The activity was measured as the formation of trichloroacetic acid-soluble [3H]inositol phosphates. No acid-soluble radioactivity accumulated over time in samples precipitated from untransfected P10-14 cells (not shown), and this background radioactivity was subtracted from the experimental samples. Shown is the mean ± standard error of the mean of five separate experiments.
Similar articles
- Pleiotropic contributions of phospholipase C-gamma1 (PLC-gamma1) to T-cell antigen receptor-mediated signaling: reconstitution studies of a PLC-gamma1-deficient Jurkat T-cell line.
Irvin BJ, Williams BL, Nilson AE, Maynor HO, Abraham RT. Irvin BJ, et al. Mol Cell Biol. 2000 Dec;20(24):9149-61. doi: 10.1128/MCB.20.24.9149-9161.2000. Mol Cell Biol. 2000. PMID: 11094067 Free PMC article. - A dynamic constitutive and inducible binding of c-Cbl by PLCgamma1 SH3 and SH2 domains (negatively) regulates antigen receptor-induced PLCgamma1 activation in lymphocytes.
Rellahan BL, Graham LJ, Tysgankov AY, DeBell KE, Veri MC, Noviello C, Bonvini E. Rellahan BL, et al. Exp Cell Res. 2003 Sep 10;289(1):184-94. doi: 10.1016/s0014-4827(03)00260-x. Exp Cell Res. 2003. PMID: 12941616 - The amino-terminal Src homology 2 domain of phospholipase C gamma 1 is essential for TCR-induced tyrosine phosphorylation of phospholipase C gamma 1.
Stoica B, DeBell KE, Graham L, Rellahan BL, Alava MA, Laborda J, Bonvini E. Stoica B, et al. J Immunol. 1998 Feb 1;160(3):1059-66. J Immunol. 1998. PMID: 9570517 - Conformational switches that control the TEC kinase - PLCγ signaling axis.
Lowe J, Joseph RE, Andreotti AH. Lowe J, et al. J Struct Biol X. 2022 Jan 22;6:100061. doi: 10.1016/j.yjsbx.2022.100061. eCollection 2022. J Struct Biol X. 2022. PMID: 35128378 Free PMC article. Review. - Emerging roles of PLCγ1 in endothelial biology.
Chen D, Simons M. Chen D, et al. Sci Signal. 2021 Aug 3;14(694):eabc6612. doi: 10.1126/scisignal.abc6612. Sci Signal. 2021. PMID: 34344833 Free PMC article. Review.
Cited by
- Dynamics of allosteric regulation of the phospholipase C-γ isozymes upon recruitment to membranes.
Siraliev-Perez E, Stariha JTB, Hoffmann RM, Temple BRS, Zhang Q, Hajicek N, Jenkins ML, Burke JE, Sondek J. Siraliev-Perez E, et al. Elife. 2022 Jun 16;11:e77809. doi: 10.7554/eLife.77809. Elife. 2022. PMID: 35708309 Free PMC article. - Structural basis for the activation of PLC-γ isozymes by phosphorylation and cancer-associated mutations.
Hajicek N, Keith NC, Siraliev-Perez E, Temple BR, Huang W, Zhang Q, Harden TK, Sondek J. Hajicek N, et al. Elife. 2019 Dec 31;8:e51700. doi: 10.7554/eLife.51700. Elife. 2019. PMID: 31889510 Free PMC article. - Phospholipase C and D regulation of Src, calcium release and membrane fusion during Xenopus laevis development.
Stith BJ. Stith BJ. Dev Biol. 2015 May 15;401(2):188-205. doi: 10.1016/j.ydbio.2015.02.020. Epub 2015 Mar 5. Dev Biol. 2015. PMID: 25748412 Free PMC article. Review. - A double point mutation in PCL-gamma1 (Y509A/F510A) enhances Y783 phosphorylation and inositol phospholipid-hydrolyzing activity upon EGF stimulation.
Chung SH, Kim SK, Kim JK, Yang YR, Suh PG, Chang JS. Chung SH, et al. Exp Mol Med. 2010 Mar 31;42(3):216-27. doi: 10.3858/emm.2010.42.3.023. Exp Mol Med. 2010. PMID: 20164676 Free PMC article. - RIAM regulates the cytoskeletal distribution and activation of PLC-gamma1 in T cells.
Patsoukis N, Lafuente EM, Meraner P, Kim Js, Dombkowski D, Li L, Boussiotis VA. Patsoukis N, et al. Sci Signal. 2009 Dec 1;2(99):ra79. doi: 10.1126/scisignal.2000409. Sci Signal. 2009. PMID: 19952372 Free PMC article.
References
- Bar-Sagi D, Rotin D, Batzer A, Mandiyan V, Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993;74:83–91. - PubMed
- Carpenter G, Hernandez-Sotomayor S M, Nishibe S, Todderud G, Mumby M, Wahl M. Growth factor phosphorylation of PLC-gamma 1. Ciba Found Symp. 1992;164:223–233. - PubMed
- Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous