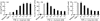Counter-regulatory effect of sodium butyrate on tumour necrosis factor-alpha (TNF-alpha)-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells - PubMed (original) (raw)
Counter-regulatory effect of sodium butyrate on tumour necrosis factor-alpha (TNF-alpha)-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells
A Andoh et al. Clin Exp Immunol. 1999 Oct.
Abstract
The various biological activities of butyrate have been well documented. In this study, we tested the effects of butyrate on TNF-alpha-induced complement C3 and factor B biosynthesis in human intestinal epithelial cells. The biosynthesis of C3, factor B and IL-8 was evaluated at the protein and mRNA levels. To evaluate transcriptional activation, the nuclear run-on assay was performed. The transcription factor-DNA binding activity was assessed by an electrophoretic gel mobility shift assay (EMSA). In the intestinal epithelial cell lines HT-29, T84 and Caco-2, sodium butyrate enhanced TNF-alpha-induced C3 secretion, but suppressed TNF-alpha-induced factor B and IL-8 secretion. Nuclear run-on assay revealed that transcriptional regulatory mechanisms are involved in the effects of sodium butyrate. The EMSAs indicated that sodium butyrate suppressed TNF-alpha-induced nuclear factor (NF)-kappaB- and activation protein (AP)-1-DNA binding activity, but enhanced TNF-alpha-induced activation of CCAAT/enhancer-binding protein (C/EBP)beta (NF-IL-6)-DNA binding activity. Sodium butyrate induced a counter-regulatory effect on TNF-alpha-induced C3 and factor B biosynthesis in human intestinal epithelial cells. Butyrate action has been discussed with its activity to induce histone hyperacetylation, but its counter-regulatory effect on complement biosynthesis may be closely associated with the modulation of transcription factor activation.
Figures
Fig. 1
Effects of sodium butyrate on TNF-α-induced C3 (a), factor B (b) and IL-8 (c) secretion in HT-29 cells. Cells were incubated with medium alone, TNF-α (25 ng/ml), or TNF-α (25 ng/ml) plus increasing concentrations of sodium butyrate for 24 h. The amounts of C3, factor B and IL-8 were determined by ELISA. Values were expressed as mean ± s.d. of quadruplicate cultures.
Fig. 2
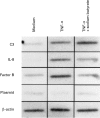
Effects of sodium butyrate on TNF-α-induced C3 (a) and factor B (b) secretion in T84 and Caco-2 cells. Cells were incubated with medium alone, sodium butyrate 5 m
m
, TNF-α 25 ng/ml, or sodium butyrate 5 m
m
plus TNF-α 25 ng/ml for 24 h. The amounts of C3 and factor B were determined by ELISA. Values were expressed as mean ± s.d. of quadruplicate cultures. □, T84; ▪, Caco-2.
Fig. 3
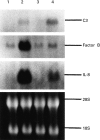
Northern blot analysis of C3, factor B and IL-8 mRNA expression in HT-29 cells. Cells were incubated with medium alone, TNF-α 25 ng/ml, sodium butyrate 5 m
m
, or TNF-α 25 ng/ml plus sodium butyrate 5 m
m
for 6 h, and then total cellular RNA was extracted. Lane 1, medium alone; lane 2, TNF-α; lane 3, sodium butyrate 5 m
m
; lane 4, TNF-α 25 ng/ml plus sodium butyrate 5 m
m
.
Fig. 4
Nuclear run-on assays of C3, IL-8 and factor B gene transcription in HT-29 cells. Confluent HT-29 monolayers were stimulated with TNF-α 25 ng/ml or TNF-α 25 ng/ml plus sodium butyrate 5 m
m
for 3 h, and then nuclei were prepared. Run-on transcription was performed in 5.0 × 106 nuclei in the presence of α-32P-uridine triphosphate for 60 min, and nuclear RNA was isolated and hybridized to filter-bound DNA probe. Plasmid, a 2.9-kb empty plasmid (pAT153/Pvu) isolated from human C3 cDNA clone pHLC3.11.
Fig. 5
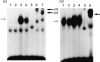
Electrophoretic gel mobility shift assays (EMSAs) for NF-κB (a) and AP-1 (b) DNA binding activities. HT-29 cells were incubate with medium alone, sodium butyrate 5 m
m
, TNF-α 25 ng/ml, or sodium butyrate 5 m
m
plus TNF-α 25 ng/ml for 1.5 h, and then nuclear extracts were prepared. (a) Lane 1, medium alone; lane 2, sodium butyrate; lane 3, TNF-α; lane 4, sodium butyrate plus TNF-α; lane 5, TNF-α plus cold probe; lane 6, TNF-α plus anti-p50 antibody; lane 7, TNF-α plus anti-p65 antibody. (b) Lane 1, medium alone; lane 2, sodium butyrate; lane 3, TNF-α; lane 4, sodium butyrate plus TNF-α; lane 5, TNF-α plus cold probe; lane 6, TNF-α plus anti-c-jun antibody. Thin arrow on the left indicates specific band, and thick arrow on the right indicates supershift band.
Fig. 6
Electrophoretic gel mobility shift assays (EMSAs) for C/EBP (a) and Sp1 (b) DNA binding activities. HT-29 cells were incubate with medium alone, sodium butyrate 5 m
m
, TNF-α 25 ng/ml, or sodium butyrate 5 m
m
plus TNF-α 25 ng/ml for 1.5 h, and then nuclear extracts were prepared. (a) Lane 1, medium alone; lane 2, sodium butyrate; lane 3, TNF-α; lane 4, sodium butyrate plus TNF-α; lane 5, TNF-α plus cold probe; lane 6, TNF-α plus anti-C/EBP α antibody; lane 7, TNF-α plus anti-C/EBPβ (NF-IL-6) antibody; lane 8, TNF-α plus anti-C/EBP δ antibody. (b) Lane 1, medium alone; lane 2, sodium butyrate; lane 3, TNF-α; lane 4, sodium butyrate plus TNF-α; lane 5, TNF-α plus cold probe; lane 6, TNF-α sample plus anti-Sp1 antibody. Thin arrow on the left indicates specific band, and thick arrow on the right indicates supershift band.
References
- Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992:411–52. - PubMed
- Braegger CP, Nicholls SW, Murch SH, et al. Tumor necrosis factor alpha in stool as a marker of intestinal inflammation. Lancet. 1992;339:89–91. - PubMed
- Breese EJ, Michte CA, Nicholls SW, et al. Tumor necrosis factor α-producing cells in the intestinal mucosa of children with inflammatory bowel disease. Gastroenterology. 1994;106:1455–66. - PubMed
- Morgan BP. The complement system. In: Morgan BP, editor. Complement: clinical aspects and relevance to disease. London: Academic Press; 1990. pp. 1–35.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous