Ras regulates sympathetic neuron survival by suppressing the p53-mediated cell death pathway - PubMed (original) (raw)
Ras regulates sympathetic neuron survival by suppressing the p53-mediated cell death pathway
I E Mazzoni et al. J Neurosci. 1999.
Abstract
In this report, we examine how the Ras protein regulates neuronal survival, focusing on sympathetic neurons. Adenovirus-expressed constitutively activated Ras (RasV12) enhanced survival and the phosphorylation of Akt (protein kinase B) and MAP kinase (MAPK), two targets of Ras activity. Functional inhibition of endogenous Ras by adenovirus-expressed dominant-inhibitory Ras (N17Ras) decreased nerve growth factor (NGF)-dependent survival and both Akt and MAPK phosphorylation as well. To determine the signaling pathways through which Ras mediates survival, we used Ras effector mutants and pharmacological inhibitors that selectively suppress phosphatidylinositol 3-kinase (PI3-K)/Akt or MAP kinase kinase (MEK)/MAPK pathways. The Ras effector mutant Ras(V12)Y40C, which selectively stimulates PI3-K and Akt, rescued survival in the absence of NGF, and the PI3-K inhibitor LY 294002 inhibited both Ras- and NGF-dependent survival. Ras(V12)T(35)S, which activates MEK/MAPK but not PI3-K/Akt, was less effective at rescuing survival, whereas the MEK inhibitor PD 098059 also partially suppressed Ras-dependent survival. To investigate the mechanisms by which Ras suppresses neuronal death, we examined whether Ras functions by inhibiting the proapoptotic p53 pathway (Jun-N-terminal kinase/p53/BAX) that is necessary for neuronal death after NGF withdrawal and p75NTR activation. We found that RasV12 suppressed c-jun, BAX, and p53 levels, whereas inhibition of NGF-induced Ras-survival activity via N17Ras increased the levels of these proteins. Furthermore, the E1B55K protein, which suppresses p53 activity, blocked N17Ras-induced neuronal death. Together, these results indicate that Ras is, in part, both necessary and sufficient for survival of sympathetic neurons and that this effect is mediated by activation of both the PI3-K- and MEK-signaling cascades, which in turn suppress a proapoptotic p53 pathway.
Figures
Fig. 1.
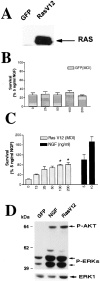
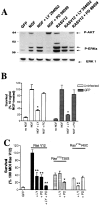
Ras is sufficient to sustain sympathetic neuronal survival and stimulates the phosphorylation of Akt and MAPK.A, Expression of RasV12 in sympathetic neurons is shown. NGF-selected sympathetic neurons were infected with 100 MOI of recombinant adenovirus-expressing GFP or RasV12; 3 d later, neurons were lysed, and equal amounts of protein were analyzed by Western blotting with an antibody specific for Ras. Note the high level of expression of RasV12. B, C, Ras is sufficient to prevent apoptotic neuronal death after NGF withdrawal. Sympathetic neurons were cultured for 5 d in the presence of 50 ng/ml NGF and infected with different MOIs of recombinant adenoviruses expressing either GFP (B) or RasV12 (C). Forty-eight hours after the onset of the infection, NGF was withdrawn for 2 d, and survival was assessed using MTT assays. As controls, uninfected sister cultures were maintained in 5 or 10 ng/ml NGF for the final 2 d (black bars in C). Each point represents the mean ± SEM of quadruplicate wells from three representative experiments. Results are normalized to the amount of survival obtained with 5 ng/ml NGF. Values for RasV12 are significantly different from that of control (NGF-deprived) cells at_p_ < 0.05 (*) (one-way ANOVA and_post_ hoc Dunnett's multiple comparison test). D, RasV12 sustains high levels of phosphorylation of Akt and MAPK in the absence of NGF. After 5 d in 50 ng/ml NGF, cultures were infected with 100 MOI of RasV12 or GFP control virus. Twenty-four and forty-eight hours after the onset of the infection, cells were deprived of NGF. As a positive control, cultures were maintained in the continuous presence of 10 ng/ml NGF. After 2 d, cells were lysed, and Western blot analyses were performed using phosphorylation and activation state-specific antibodies against Akt or MAPK. Note that the levels of phosphorylation of these two Ras downstream effectors are similar in cultures infected with RasV12 and in those treated with NGF. To confirm that the amounts of protein were similar in each_lane_, we reprobed the blots with anti-ERK1 antibody (bottom bands). P-AKT, Phospho-Akt;P-ERKs, phospho-MAPKs.
Fig. 2.
Immunohistochemistry of Ras mutants expressed in sympathetic neurons via recombinant adenovirus. Sympathetic neurons were infected with 100 MOI of c-myc-tagged RasV12 (A,C) or 200 MOI of c-myc-tagged N17Ras (B,D) in the presence of NGF, and 48 hr later cells were processed for immunocytochemistry using anti-myc (A,B). Note that the majority of neurons (phase contrast in_C,_ D) express the Ras mutant proteins.
Fig. 3.
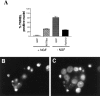
Ras regulates sympathetic neuron apoptosis.A, Quantitation of TUNEL-positive cells is shown. Cultures (n = 3) were infected with RasV12 (100 MOI) or N17Ras (200 MOI) in the presence of 10 ng/ml NGF. Twenty-four hours after infection, cultures infected with RasV12 were deprived of NGF, while those infected with N17Ras were maintained in 10 ng/ml NGF. Three days after infection, cells were processed for TUNEL and Hoechst staining. As controls, sympathetic neurons were infected with 200 MOI (control for N17 Ras) or 100 MOI (control for RasV12) of a recombinant adenovirus expressing GFP. Results are expressed as a percentage of TUNEL-positive neurons relative to the total number of Hoechst-labeled cells. B, C, Sympathetic neurons infected with N17Ras (200 MOI) and maintained in 10 ng/ml NGF for 3 d after infection were processed for TUNEL (B) and Hoechst staining (C). As determined by fluorescence microscopy, neurons that were TUNEL positive (B) had nuclei containing highly condensed or fragmented chromatin (C), confirming that they were undergoing apoptosis, whereas those that were TUNEL negative always had spherical and uniformly distributed chromatin.
Fig. 4.
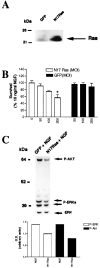
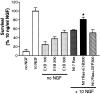
Dominant-inhibitory Ras suppresses neurotrophin-mediated sympathetic neuron survival. _A,_Expression of dominant-inhibitory Ras in sympathetic neurons is shown. NGF-selected sympathetic neurons were infected with adenovirus expressing GFP or N17Ras; 3 d later, neurons were lysed, and equal amounts of protein were separated by gel electrophoresis. After transfer, the blot was probed with anti-Ras. Note that endogenous Ras can be seen in the GFP-infected neurons (arrow), but that N17Ras is expressed at much higher levels. As reported previously, Ras migrates at a slightly higher apparent molecular weight than 21 kDa (Cox et al., 1995). B, MTT assays for sympathetic neurons infected with various MOIs of recombinant adenovirus expressing either N17Ras or GFP in the presence of NGF are shown. Neurons were selected in 50 ng/ml NGF for 5 d, infected with adenovirus, and, 48 hr after the onset of the infection, switched into media containing 10 ng/ml NGF for an additional 2 d, before the MTT assay was performed. Values are normalized to that of 10 ng/ml NGF, which supports 100% neuronal survival (Belliveau et al., 1997), and error bars represent the SEM. A value that is statistically different from that of 10 ng/ml NGF is denoted by an * at p< 0.01(one-way ANOVA and post _hoc_Dunnett's multiple comparison test). Results are the means from three independent experiments. The GFP adenovirus has no effect on neuronal survival, whereas N17Ras significantly reduces NGF-mediated survival. C, Top, N17Ras decreases the levels of phosphorylation of Akt and MAPK in cultures maintained in NGF. After 5 d in 50 ng/ml NGF, cultures were infected with N17Ras or GFP control virus (200 MOI) for 24 hr in the presence of 10 ng/ml NGF. Three days later, cells were lysed, and equal amounts of proteins were analyzed by Western blotting using phosphorylation and activation state-specific antibodies against Akt or MAPK. Blots were reprobed with an antibody directed against total ERK1 (bottom bands). Shown is a representative blot of the experiment performed at least three times.Bottom, The intensity of each band (top), normalized against those of total ERK1, is shown in the bar graph. Note the decrease in the levels of NGF-mediated phosphorylation of both phospho-Akt (P-Akt) and, to a lesser extent, phospho-MAPK (P-ERK) in cultures infected with N17Ras.
Fig. 5.
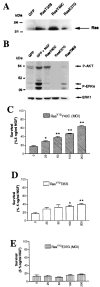
Ras sustains sympathetic neuronal survival via multiple signaling pathways. A, Expression of Ras effector mutants in sympathetic neurons is shown. Cells were infected with 100 MOI of adenoviruses expressing GFP, RasV12T35S (RasT35S), or RasV12E37G (RasE37G) or with 200 MOI of RasV12Y40C (RasY40C) adenovirus for 3 d. Equivalent amounts of protein were separated by gel electrophoresis, as shown by the bottom bands, and Western blots were probed with anti-Ras. Note that the levels of expression of the different Ras effector mutants are comparable. B, Ras effector mutants differentially activate Ras downstream targets. Sympathetic neurons were infected with 200 MOI of RasV12Y40C, 100 MOI of RasV12T35S, or 100 MOI of RasV12E37G in the presence of 30 ng/ml NGF, and 48 hr after the onset of the infection, NGF was withdrawn. As controls, neurons were infected with 100 MOI of GFP adenovirus and maintained in 10 ng/ml NGF (GFP + NGF). Two days after NGF withdrawal, cell lysates were prepared and normalized for equal amounts of proteins. Phosphorylation of MAPK and Akt was examined on Western blots using anti-phospho-MAPK (P-ERK) or anti-phospho-Akt (P-AKT). Note that RasV12Y40C only induces phosphorylation of Akt, whereas RasV12T35S is able to induce phosphorylation of MAPK (ERK) but not Akt. As expected, RasV12E37G did not stimulate the phosphorylation of these two Ras downstream effectors. C–E, Ras sustains sympathetic neuron survival via PI3-K and Raf but not RalGDS. Sympathetic neurons were prepared and after 5 d in vitro infected with increasing MOIs of adenovirus expressing RasV12Y40C (C), RasV12T35S (D), or RasV12E37G (E), which selectively activates PI3-K, Raf/MEK/MAPK, or RalGDS pathways, respectively. Results from the MTT assay performed 2 d after NGF withdrawal are expressed as the percentage of survival with 5 ng/ml NGF and are means ± SEM of quadruplicate wells from three independent experiments. Values are significantly different from that of NGF-deprived cultures (0 MOI) at_p_ < 0.05 (*) or p < 0.01 (**) (one-way ANOVA and post hoc Dunnett's multiple comparison test).
Fig. 6.
Inhibition of NGF- and Ras-mediated survival by the PI3-K inhibitor LY 294002 and the MEK inhibitor PD 98059.A, NGF-selected sympathetic neurons were infected with 100 MOI of adenoviruses expressing GFP or RasV12. Two days after the initiation of the infection, cultures were switched into media with no NGF but containing 100 μ
m
LY 294002 or 75 μ
m
PD 98059. As controls, mock-infected sister cultures were switched into media containing 10 ng/ml NGF plus one of the same two drugs. Two days later, neurons were lysed and analyzed by Western blotting with anti-phospho-Akt (P-AKT) and anti-phospho-MAPK (P-ERK). To ensure that similar amounts of proteins were present in all lanes, we reprobed the blot with an antibody against ERK1 (bottom bands). Note that, as expected, LY 294002 inhibits phosphorylation of Akt, whereas PD 98059 only affects phosphorylation of MAPK. B, C, PI3-K and MEK are required to mediate Ras survival effects on sympathetic neurons. B, Sympathetic neurons were infected with 100 MOI of GFP adenovirus and were switched to media containing 10 ng/ml NGF plus 100 μ
m
LY 294002 (LY) or 75 μ
m
PD 98059 (PD) 48 hr after the onset of the infection. MTT assays were performed 2 d later. Mock-infected sister cultures were also analyzed after the same treatment. C, Neurons were infected with 100 MOI of adenoviruses expressing GFP, RasV12, RasV12T35S, or RasV12Y40C adenovirus (200 MOI) and 48 hr later switched to media containing no NGF and 100 μ
m
LY 294002, 75 μ
m
PD 98059, or both for 2 d before MTT assays. In B and C, results represent the means ± SEM of four to five replicates from three independent experiments and are expressed as the percentage of survival attained with 10 ng/ml NGF (B) or RasV12 (C). Results are different from their appropriate control (no drug treatment) at p < 0.05 (*) or_p_ < 0.01 (**) (one-way ANOVA and_post_ hoc Dunnett's multiple comparison test).
Fig. 7.
Dominant-inhibitory Ras induces and activated Ras suppresses the p53 apoptotic pathway. Sympathetic neurons were infected with 100 MOI of RasV12 (A) or GFP (A, labeled GFP − NGF) or 200 MOI of N17Ras (B). Forty-eight hours after infection, the media were changed, and cultures were maintained in the absence (GFP − NGF,RasV12 − NGF) or presence (NGF, N17Ras + NGF) of 10 ng/ml NGF. Two days later, cells were lysed, and equal amounts of proteins were separated electrophoretically and transferred onto nitrocellulose membranes. Western blots were probed with anti c-jun, anti-p53, anti-Bax, anti- P-Akt, anti-P-ERK, and anti-ERK 1. Note that in NGF-treated cultures, N17Ras induces an increase in the levels of expression of c-jun and a shift to a larger apparent molecular weight, indicative of increased phosphorylation (B). RasV12 suppressed (A) whereas N17Ras increased the levels of p53 and Bax protein (B). Shown are representative blots of experiments performed at least four times. The_lanes_ labeled ERK 1 indicate total protein amounts assayed. P-Akt, Phospho-Akt;P-ERK, phospho-MAPK.
Fig. 8.
p53 is required for neuronal death induced by suppression of NGF-induced Ras activity. Sympathetic neurons maintained in the presence of 10 ng/ml NGF were infected with increasing MOI of E1B55K (which functionally ablates p53) and withdrawn from neurotrophin support 48 hr after infection. Neurons were also infected with 200 MOI of N17Ras either alone or with the simultaneous addition of either 300 MOI of E1B55K (E1B) or, as a control, 300 MOI of GFP and kept in the continuous presence of 10 ng/ml NGF. In all cases, neurons were processed for MTT assays 96 hr after infection. Values are normalized as the percentage of survival attained with 10 ng/ml NGF and represent means ± SEM of quadruplicate wells from three experiments. Values for N17Ras + GFP are significantly different from those for N17Ras + E1B55K at p < 0.05 (*) (one-way ANOVA and post hoc Dunnett's multiple comparison test). E1B55K significantly blocked the apoptotic effects induced by N17Ras in NGF-treated cultures.
Similar articles
- Depolarization and neurotrophins converge on the phosphatidylinositol 3-kinase-Akt pathway to synergistically regulate neuronal survival.
Vaillant AR, Mazzoni I, Tudan C, Boudreau M, Kaplan DR, Miller FD. Vaillant AR, et al. J Cell Biol. 1999 Sep 6;146(5):955-66. doi: 10.1083/jcb.146.5.955. J Cell Biol. 1999. PMID: 10477751 Free PMC article. - p53 is essential for developmental neuron death as regulated by the TrkA and p75 neurotrophin receptors.
Aloyz RS, Bamji SX, Pozniak CD, Toma JG, Atwal J, Kaplan DR, Miller FD. Aloyz RS, et al. J Cell Biol. 1998 Dec 14;143(6):1691-703. doi: 10.1083/jcb.143.6.1691. J Cell Biol. 1998. PMID: 9852160 Free PMC article. - Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons.
Crowder RJ, Freeman RS. Crowder RJ, et al. J Neurosci. 1998 Apr 15;18(8):2933-43. doi: 10.1523/JNEUROSCI.18-08-02933.1998. J Neurosci. 1998. PMID: 9526010 Free PMC article. - Rit subfamily small GTPases: regulators in neuronal differentiation and survival.
Shi GX, Cai W, Andres DA. Shi GX, et al. Cell Signal. 2013 Oct;25(10):2060-8. doi: 10.1016/j.cellsig.2013.06.002. Epub 2013 Jun 11. Cell Signal. 2013. PMID: 23770287 Free PMC article. Review. - Blockade of mutant RAS oncogenic signaling with a special emphasis on KRAS.
Roskoski R Jr. Roskoski R Jr. Pharmacol Res. 2021 Oct;172:105806. doi: 10.1016/j.phrs.2021.105806. Epub 2021 Aug 24. Pharmacol Res. 2021. PMID: 34450320 Review.
Cited by
- Deregulation of the Egfr/Ras signaling pathway induces age-related brain degeneration in the Drosophila mutant vap.
Botella JA, Kretzschmar D, Kiermayer C, Feldmann P, Hughes DA, Schneuwly S. Botella JA, et al. Mol Biol Cell. 2003 Jan;14(1):241-50. doi: 10.1091/mbc.e02-05-0297. Mol Biol Cell. 2003. PMID: 12529440 Free PMC article. - The Acquisition of Target Dependence by Developing Rat Retinal Ganglion Cells.
Moses C, Wheeler LP, LeVaillant CJ, Kramer A, Ryan M, Cozens GS, Sharma A, Pollett MA, Rodger J, Harvey AR. Moses C, et al. eNeuro. 2015 Jul 10;2(3):ENEURO.0044-14.2015. doi: 10.1523/ENEURO.0044-14.2015. eCollection 2015 May-Jun. eNeuro. 2015. PMID: 26464991 Free PMC article. - Inhibition of the Ras/Raf/ERK1/2 Signaling Pathway Restores Cultured Spinal Cord-Injured Neuronal Migration, Adhesion, and Dendritic Spine Development.
Xu D, Cao F, Sun S, Liu T, Feng S. Xu D, et al. Neurochem Res. 2016 Aug;41(8):2086-96. doi: 10.1007/s11064-016-1921-1. Epub 2016 Apr 21. Neurochem Res. 2016. PMID: 27097549 - Role of PI 3-kinase, Akt and Bcl-2-related proteins in sustaining the survival of neurotrophic factor-independent adult sympathetic neurons.
Orike N, Middleton G, Borthwick E, Buchman V, Cowen T, Davies AM. Orike N, et al. J Cell Biol. 2001 Sep 3;154(5):995-1005. doi: 10.1083/jcb.200101068. Epub 2001 Aug 27. J Cell Biol. 2001. PMID: 11524433 Free PMC article. - Multiple distinct signal pathways, including an autocrine neurotrophic mechanism, contribute to the survival-promoting effect of depolarization on spiral ganglion neurons in vitro.
Hansen MR, Zha XM, Bok J, Green SH. Hansen MR, et al. J Neurosci. 2001 Apr 1;21(7):2256-67. doi: 10.1523/JNEUROSCI.21-07-02256.2001. J Neurosci. 2001. PMID: 11264301 Free PMC article.
References
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–868. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous