CD2 sets quantitative thresholds in T cell activation - PubMed (original) (raw)
CD2 sets quantitative thresholds in T cell activation
M F Bachmann et al. J Exp Med. 1999.
Abstract
It has been proposed that CD2, which is highly expressed on T cells, serves to enhance T cell-antigen presenting cell (APC) adhesion and costimulate T cell activation. Here we analyzed the role of CD2 using CD2-deficient mice crossed with transgenic mice expressing a T cell receptor specific for lymphocytic choriomeningitis virus (LCMV)-derived peptide p33. We found that absence of CD2 on T cells shifted the p33-specific dose-response curve in vitro by a factor of 3-10. In comparison, stimulation of T cells in the absence of lymphocyte function-associated antigen (LFA)-1-intercellular adhesion molecule (ICAM)-1 interaction shifted the dose-response curve by a factor of 10, whereas absence of both CD2-CD48 and LFA-1-ICAM-1 interactions shifted the response by a factor of approximately 100. This indicates that CD2 and LFA-1 facilitate T cell activation additively. T cell activation at low antigen density was blocked at its very first steps, as T cell APC conjugate formation, TCR triggering, and Ca(2+) fluxes were affected by the absence of CD2. In vivo, LCMV-specific, CD2-deficient T cells proliferated normally upon infection with live virus but responded in a reduced fashion upon cross-priming. Thus, CD2 sets quantitative thresholds and fine-tunes T cell activation both in vitro and in vivo.
Figures
Figure 1
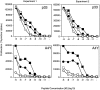
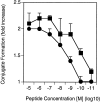
CD2–CD48 and LFA-1–ICAM-1 interactions enhance T cell proliferation at low peptide densities. Thioglycollate-elicited macrophages derived from control (filled symbols) or ICAM-1–deficient (open symbols) mice were pulsed with various doses of peptide p33 or the low-affinity ligand A4Y and used to stimulate T cells derived from TCR-transgenic control (squares) or CD2-deficient (circles) mice. Proliferation was assessed 36 h later by means of [3H]thymidine incorporation. Two independent experiments are shown. ▪, CD2+ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
Figure 3
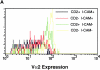
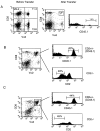
CD2–CD48 and LFA-1–ICAM-1 enhance T cell activation by altering signal 1. Thioglycollate-elicited macrophages derived from control or ICAM-1–deficient mice were pulsed with various doses of peptide p33, mixed with T cells derived from TCR-transgenic control or CD2-deficient mice, and centrifuged together. Expression of TCR (Vα2) was assessed 4 h later on CD8+ T cells. (A) TCR expression is shown for various combinations after stimulation with 10−10 M p33-pulsed macrophages. (B) Mean fluorescence of TCR expression is shown as a function of the peptide concentration for the various combinations. One representative experiment of three is shown. ▪, CD2+ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
Figure 3
CD2–CD48 and LFA-1–ICAM-1 enhance T cell activation by altering signal 1. Thioglycollate-elicited macrophages derived from control or ICAM-1–deficient mice were pulsed with various doses of peptide p33, mixed with T cells derived from TCR-transgenic control or CD2-deficient mice, and centrifuged together. Expression of TCR (Vα2) was assessed 4 h later on CD8+ T cells. (A) TCR expression is shown for various combinations after stimulation with 10−10 M p33-pulsed macrophages. (B) Mean fluorescence of TCR expression is shown as a function of the peptide concentration for the various combinations. One representative experiment of three is shown. ▪, CD2+ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
Figure 2
CD2–CD48 and LFA-1–ICAM-1 interaction enhances IFN-γ production at low peptide densities. Thioglycollate-elicited macrophages derived from control (filled symbols) or ICAM-1–deficient (open symbols) mice were pulsed with various doses of peptide p33 or the low-affinity ligand A4Y and used to stimulate T cells derived from TCR-transgenic control (squares) or CD2-deficient (circles) mice. Production of IFN-γ was assessed by ELISA 3 d later from culture supernatants. Two independent representative experiments are shown. ▪, CD2+ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
Figure 4
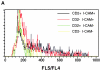
CD2–CD48 and LFA-1–ICAM-1 enhance Ca2+ fluxes at low antigen concentration. Thioglycollate-elicited macrophages derived from control or ICAM-1–deficient mice were pulsed with various doses of peptide p33, mixed with INDO-1–pulsed, purified CD8+ T cells derived from TCR-transgenic control or CD2-deficient mice, and centrifuged together. Elevation of [Ca2+]i was assessed by measuring the FL5/FL4 ratio. (A) FL5/FL4 ratio is shown after stimulation with 10−11 M p33-pulsed macrophages. (B) Mean FL5/FL4 ratios are shown as a function of the peptide concentration for the various combinations. Baseline FL5/FL4 values were subtracted for the calculation. One representative experiment of two is shown. ▪, CD2+ ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
Figure 4
CD2–CD48 and LFA-1–ICAM-1 enhance Ca2+ fluxes at low antigen concentration. Thioglycollate-elicited macrophages derived from control or ICAM-1–deficient mice were pulsed with various doses of peptide p33, mixed with INDO-1–pulsed, purified CD8+ T cells derived from TCR-transgenic control or CD2-deficient mice, and centrifuged together. Elevation of [Ca2+]i was assessed by measuring the FL5/FL4 ratio. (A) FL5/FL4 ratio is shown after stimulation with 10−11 M p33-pulsed macrophages. (B) Mean FL5/FL4 ratios are shown as a function of the peptide concentration for the various combinations. Baseline FL5/FL4 values were subtracted for the calculation. One representative experiment of two is shown. ▪, CD2+ ICAM+; •, CD2−ICAM+; □, CD2+ICAM−; ○, CD2−ICAM−.
Figure 5
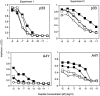
CD2–CD48 interaction enhances T cell–APC conjugate formation. Thioglycollate-elicited macrophages were pulsed with various doses of peptide p33, mixed with INDO-1–pulsed, purified CD8+ T cells derived from TCR-transgenic control (▪) or CD2-deficient (•) mice, and centrifuged together, and conjugate formation was assessed. Data from two independent experiments were pooled, and the average and SD is shown.
Figure 7
Normal in vivo expansion of LCMV-GP–specific, CD2-deficient, TCR-transgenic T cells upon infection with live virus. CD45.1+ TCR-transgenic control spleen cells were mixed 1:1 with CD45.1+ CD2-deficient spleen cells and adoptively transferred into C57BL/6 recipient mice. (A) Left panel: 1:1 distribution of CD2+ and CD2− TCR+ T cells was confirmed before transfer. Center and right panels: splenocytes from mice that received 106 cells of the mixture shown in the left panel 6 d earlier in the absence of an infection were stained for Vα2, CD8, and CD45.1 expression; <2% of CD8+Vα2+ T cells were derived from the adoptively transferred T cells. (B and C) Recipient mice were infected with LCMV (200 PFU; B) or recombinant vaccinia virus expressing LCMV-GP (2 × 106 PFU; C). CD45.1 expression was assessed for CD8+Vα2+ T cells, revealing expansion of CD2+ control T cells (upper right panels). CD2 expression was assessed similarly for CD8+Vα2+ T cells, revealing expansion of CD2-deficient T cells (lower right panels). Similar results were obtained 8 d after infection. One representative experiment of three is shown.
Figure 6
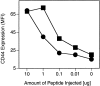
CD2 reduces the amount of peptide required for in vivo induction of CD44 expression. TCR-transgenic control (▪) and CD2-deficient (•) mice were injected with various amounts of peptide p33, and expression of CD44 was assessed 24 h later on CD8+Vα2+ T cells. The average of two mice is shown per dose. One representative experiment of three is shown.
Figure 8
Impaired in vivo expansion of LCMV-GP–specific, TCR-transgenic, CD2-deficient T cells upon cross-priming. CD45.1+ TCR-transgenic control spleen cells were mixed 1:1 with CD45.1+ CD2-deficient spleen cells and adoptively transferred into C57BL/6 recipient mice. (A) Recipient mice were immunized with UV light–inactivated recombinant vaccinia virus expressing LCMV-GP, and spleen cells were analyzed 6 d later. CD45.1 expression was assessed for CD8+Vα2+ T cells, revealing expansion of CD2+ control T cells (upper right panels). CD2 expression was assessed similarly for CD8+ Vα2+ T cells, revealing expansion of CD2-deficient T cells (lower right panels). (B) Recipient mice were immunized with LCMV-GP associated with cellular debris, and spleen cells were analyzed 6 d later. CD45.1 expression was assessed for CD8+Vα2+ T cells, revealing expansion of CD2+ control T cells (upper right panels). CD2 expression was assessed similarly for CD8+Vα2+ T cells, revealing expansion of CD2-deficient T cells (lower right panels). Percentage of CD45.1+ control T cells versus CD2-deficient T cells was <3% in the absence of immunization. One representative experiment of two is shown.
Comment in
- A subtle role for CD2 in T cell antigen recognition.
van der Merwe PA. van der Merwe PA. J Exp Med. 1999 Nov 15;190(10):1371-4. doi: 10.1084/jem.190.10.1371. J Exp Med. 1999. PMID: 10562312 Free PMC article. No abstract available.
Similar articles
- Coengagement of CD2 with LFA-1 or VLA-4 by bispecific ligand fusion proteins primes T cells to respond more effectively to T cell receptor-dependent signals.
Dietsch MT, Chan PY, Kanner SB, Gilliland LK, Ledbetter JA, Linsley PS, Aruffo A. Dietsch MT, et al. J Leukoc Biol. 1994 Oct;56(4):444-52. doi: 10.1002/jlb.56.4.444. J Leukoc Biol. 1994. PMID: 7523557 - A subtle role for CD2 in T cell antigen recognition.
van der Merwe PA. van der Merwe PA. J Exp Med. 1999 Nov 15;190(10):1371-4. doi: 10.1084/jem.190.10.1371. J Exp Med. 1999. PMID: 10562312 Free PMC article. No abstract available. - Remote T cell co-stimulation via LFA-1/ICAM-1 and CD2/LFA-3: demonstration with immobilized ligand/mAb and implication in monocyte-mediated co-stimulation.
Van Seventer GA, Shimizu Y, Horgan KJ, Luce GE, Webb D, Shaw S. Van Seventer GA, et al. Eur J Immunol. 1991 Jul;21(7):1711-8. doi: 10.1002/eji.1830210719. Eur J Immunol. 1991. PMID: 1711977 - The CD2-LFA-3 and LFA-1-ICAM pathways: relevance to T-cell recognition.
Makgoba MW, Sanders ME, Shaw S. Makgoba MW, et al. Immunol Today. 1989 Dec;10(12):417-22. doi: 10.1016/0167-5699(89)90039-X. Immunol Today. 1989. PMID: 2482743 Review. - T Cell Activation Pathways: B7, LFA-3, and ICAM-1 Shape Unique T Cell Profiles.
Wingren AG, Parra E, Varga M, Kalland T, Sjogren HO, Hedlund G, Dohlsten M. Wingren AG, et al. Crit Rev Immunol. 2017;37(2-6):463-481. doi: 10.1615/CritRevImmunol.v37.i2-6.130. Crit Rev Immunol. 2017. PMID: 29773030 Review.
Cited by
- Stepwise progression of β-selection during T cell development involves histone deacetylation.
Chann AS, Charnley M, Newton LM, Newbold A, Wiede F, Tiganis T, Humbert PO, Johnstone RW, Russell SM. Chann AS, et al. Life Sci Alliance. 2022 Oct 25;6(1):e202201645. doi: 10.26508/lsa.202201645. Print 2023 Jan. Life Sci Alliance. 2022. PMID: 36283704 Free PMC article. - The Role of Co-Signaling Molecules in Psoriasis and Their Implications for Targeted Treatment.
Liu S, Xu J, Wu J. Liu S, et al. Front Pharmacol. 2021 Jul 20;12:717042. doi: 10.3389/fphar.2021.717042. eCollection 2021. Front Pharmacol. 2021. PMID: 34354596 Free PMC article. Review. - Antigen discrimination by T cells relies on size-constrained microvillar contact.
Jenkins E, Körbel M, O'Brien-Ball C, McColl J, Chen KY, Kotowski M, Humphrey J, Lippert AH, Brouwer H, Santos AM, Lee SF, Davis SJ, Klenerman D. Jenkins E, et al. Nat Commun. 2023 Mar 23;14(1):1611. doi: 10.1038/s41467-023-36855-9. Nat Commun. 2023. PMID: 36959206 Free PMC article. - Using CombiCells, a platform for titration and combinatorial display of cell surface ligands, to study T-cell antigen sensitivity modulation by accessory receptors.
Patel A, Andre V, Eguiguren SB, Barton MI, Burton J, Denham EM, Pettmann J, Mørch AM, Kutuzov MA, Siller-Farfán JA, Dustin ML, van der Merwe PA, Dushek O. Patel A, et al. EMBO J. 2024 Jan;43(1):132-150. doi: 10.1038/s44318-023-00012-1. Epub 2023 Dec 18. EMBO J. 2024. PMID: 38177315 Free PMC article. - Functional anatomy of T cell activation and synapse formation.
Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, Waite J, Sacristán C, Victora GD, Zanin-Zhorov A, Dustin ML. Fooksman DR, et al. Annu Rev Immunol. 2010;28:79-105. doi: 10.1146/annurev-immunol-030409-101308. Annu Rev Immunol. 2010. PMID: 19968559 Free PMC article. Review.
References
- Springer T.A., Dustin M.L., Kishimoto T.K., Marlin S.D. The lymphocyte function-associated LFA-1, CD2 and LFA-3 moleculescell adhesion receptors for the immune system. Annu. Rev. Immunol. 1987;5:223–252. - PubMed
- Bachmann M.F., McKall-Faienza K., Schmits R., Bouchard D., Beach J., Speiser D.E., Mak T.W., Ohashi P.S. Distinct roles for LFA-1 and CD28 during activation of naive T cellsadhesion versus costimulation. Immunity. 1997;7:549–557. - PubMed
- Viola A., Lanzavecchia A. T cell activation determined by T cell receptor number and tunable thresholds. Science. 1996;273:104–106. - PubMed
- Valitutti S., Müller S., Cella M., Padovan E., Lanzavecchia A. Serial triggering of many T cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous