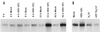The disappearance of cyclins A and B and the increase in activity of the G(2)/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the alpha22/U(S)1.5 and U(L)13 viral genes - PubMed (original) (raw)
The disappearance of cyclins A and B and the increase in activity of the G(2)/M-phase cellular kinase cdc2 in herpes simplex virus 1-infected cells require expression of the alpha22/U(S)1.5 and U(L)13 viral genes
S J Advani et al. J Virol. 2000 Jan.
Abstract
In uninfected cells the G(2)/M transition is regulated by cyclin kinase complex containing cdc2 and, initially, cyclin A, followed by cyclin B. cdc2 is downregulated through phosphorylation by wee-1 and myt-1 and upregulated by cdc-25C phosphatase. We have examined the accumulation and activities of these proteins in cells infected with wild type and mutants of herpes simplex virus 1. The results were as follows. (i) Cyclin A and B levels were reduced beginning 4 h after infection and were undetectable at 12 to 16 h after infection. (ii) cdc2 protein also decreased in amount but was detectable at all times after infection. In addition, a fraction of cdc2 protein from infected cells exhibited altered electrophoretic mobility in denaturing gels. (iii) The levels of cdk7 or myt-1 proteins remained relatively constant throughout infection, whereas the level of wee-1 was significantly decreased. (iv) cdc-25C formed novel bands characterized by slower electrophoretic mobility that disappeared after treatment with phosphatase. In addition, one phosphatase-sensitive band reacted with MPM-2 antibody that recognizes a phosphoepitope phosphorylated exclusively in M phase. (v) cdc2 accumulating in infected cells exhibited kinase activity. The activity of cdc2 was higher in infected cell lysates than those of corresponding proteins present in lysates of mock-infected cells even though cyclins A and B were not detectable in lysates of infected cells. (vi) The decrease in the levels of cyclins A and B, the increase in activity of cdc2, and the hyperphosphorylation of cdc-25C were mediated by U(L)13 and alpha22/U(S)1.5 gene products. In light of its normal functions, the activated cdc2 kinase may play a role in the changes in the morphology of the infected cell. These results are consistent with the accruing evidence that herpes simplex virus scavenges the cell for useful cell cycle proteins and subverts them for its own use.
Figures
FIG. 1
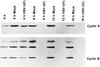
Photograph of an immunoblot of uninfected or wild-type virus-infected HeLa cell lysates electrophoretically separated on polyacrylamide gels and reacted with antibodies to cyclin A or cyclin B and then with goat anti-mouse antibody and visualized by ECL as described in Materials and Methods. The cells were infected at time zero and harvested at the indicated times after infection. Cyclin B formed three bands on electrophoresis in denaturing 10% polyacrylamide gels.
FIG. 2
Photograph showing three different exposures of one immunoblot of uninfected or wild-type virus-infected HeLa cell lysates electrophoretically separated on polyacrylamide gels and reacted with antibodies to cdc2 kinase. The procedures were as described in the legend to Fig. 1 and in Materials and Methods. The three exposures were designed to show faint bands visible at low or intermediate exposures but which become fused with the larger bands on longer exposures. Bands in mock-infected lanes are labeled a, b, and c. Bands a and b represent inhibitory phosphorylation of cdc2 in its kinase domain. cdc2 reacting bands present solely in lysates of infected cells were labeled d and e. Overexposure of the blot (lowermost strip) showed that band e accumulated in infected cells.
FIG. 3
Photograph of an immunoblot of uninfected or wild-type virus-infected HeLa cell lysates electrophoretically separated on polyacrylamide gels and reacted with antibodies to cdk7, cdc-25C, myt-1, and human wee-1 [Wee(hu)-1]. The procedures were as described above and in Materials and Methods.
FIG. 4
Photograph of an immunoblot of lysates of uninfected or wild-type-virus-infected HeLa cells treated with AP, electrophoretically separated on denaturing polyacrylamide gels, and reacted to antibodies to cdc-25C (A) or MPM-2 (B). The cells were harvested 13 h after infection. The dots mark three distinct bands of proteins reacting with antibody to cdc-25C that were no longer detected after treatment with the phosphatase. The distinct curved band (lowest dot) in infected cells reacted with antibodies to both cdc-25C and MPM-2.
FIG. 5
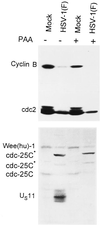
Photograph of an immunoblot of lysates of HeLa cells electrophoretically separated in denaturing gels and reacted with antibodies to cyclin B, cdc-25C, and wee-1. Replicate cultures were mock-infected or infected with HSV-1(F) and maintained in the presence of PAA (300 μg of PAA per ml). The antibody to US11 verified that PAA blocked viral DNA synthesis and precluded the synthesis of γ2 proteins dependent on the replication of viral DNA for their synthesis. Cells were incubated with or without PAA and then mock infected or infected with HSV-1(F). The cells were harvested 16 h after infection. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
FIG. 6
Photograph of an immunoblot of lysates of HeLa cells mock infected or infected with HSV-1(F), R7356 (UL13−), or R7802 (α22−/US1.5−), electrophoretically separated on polyacrylamide gels, and reacted with antibodies to cyclin A and B (A) or wee(hu)-1 and cdc-25C (B). The cells were harvested 16 h after infection. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
FIG. 7
Photograph of an immunoblot of lysates of rabbit skin cells mock infected or infected with HSV-1(F), R7356 (UL13−), R7358 (UL13 repair), R7802 (α22−/US1.5−), or R7804 (repair of R7802), electrophoretically separated on a denaturing polyacrylamide gel, and reacted with antibodies to cdc-25C. The cells were harvested 16 h after infection. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
FIG. 8
Autoradiographic image of histone H1 phosphorylated in vitro by immune precipitates of cyclin B-cdc2 complex from replicate cultures of HeLa cells mock infected or infected with HSV-1(F), R7356 (UL13−), or R7802 (α22−/US1.5−). The cyclin B-cdc2 complex was precipitated with cyclin B antibody from lysates of cells harvested 16 h after infection. The reaction mixtures consisted of the precipitate alone or precipitate plus histone H1. The mixtures were denatured and separated on denaturing polyacrylamide gels at the completion of the reaction and prior to autoradiography. The procedures were as described in the legend to Fig. 1 and in Materials and Methods.
FIG. 9
Autoradiographic image of histone H1 phosphorylated in vitro by immune precipitates of cdc2 from lysates of HeLa cells. (A) cdc2 was immune precipitated from lysates of replicate cultures of HeLa cells harvested at the intervals shown after mock infection or infection with HSV-1(F). (B) cdc2 was immune precipitated from lysates of HeLa cells harvested 16 h after mock infection of infection with HSV-1(F), R7356 (UL13−), or R7802 (α22−/US1.5−). The procedures were as described in the legend to Fig. 8 and in Materials and Methods.
FIG. 10
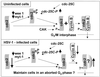
Schematic diagram of the activation of cdc2 in uninfected cells (upper panel) and wild-type virus-infected cells (lower panel). The thick arrows indicate an increase (up) or a decrease (down) in activity. The key features shown in this report are decreased expression of the regulatory cyclins A and B and of cdc2 protein, the decrease in the levels of wee-1, the change in the phosphorylation of cdc2, and the hyperphosphorylation of cdc-25C. The cumulative effects of HSV-1 infection result in the upregulation of cdc2 activity. The experiment shown in Fig. 8 indicated that the kinase activity associated with cyclin B-cdc2 complex actually decreased in infected cells, whereas the kinase activity of precipitates obtained with antibody to cdc2 increased after infection (Fig. 9). The partner of cdc2 responsible for the higher cdc2 kinase activity in infected cells is unknown and marked with a question mark in the lower panel. CAK, cyclin-activating kinase complex.
Similar articles
- The carboxyl-terminal domain of RNA polymerase II is phosphorylated by a complex containing cdk9 and infected-cell protein 22 of herpes simplex virus 1.
Durand LO, Advani SJ, Poon AP, Roizman B. Durand LO, et al. J Virol. 2005 Jun;79(11):6757-62. doi: 10.1128/JVI.79.11.6757-6762.2005. J Virol. 2005. PMID: 15890914 Free PMC article. - Herpes simplex virus 1 activates cdc2 to recruit topoisomerase II alpha for post-DNA synthesis expression of late genes.
Advani SJ, Weichselbaum RR, Roizman B. Advani SJ, et al. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4825-30. doi: 10.1073/pnas.0730735100. Epub 2003 Mar 28. Proc Natl Acad Sci U S A. 2003. PMID: 12665617 Free PMC article. - The interaction of herpes simplex virus 1 regulatory protein ICP22 with the cdc25C phosphatase is enabled in vitro by viral protein kinases US3 and UL13.
Smith-Donald BA, Roizman B. Smith-Donald BA, et al. J Virol. 2008 May;82(9):4533-43. doi: 10.1128/JVI.02022-07. Epub 2008 Feb 13. J Virol. 2008. PMID: 18272572 Free PMC article. - The herpes simplex virus type 1 infected cell protein 22.
Lin FS, Ding Q, Guo H, Zheng AC. Lin FS, et al. Virol Sin. 2010 Feb;25(1):1-7. doi: 10.1007/s12250-010-3080-x. Epub 2010 Feb 12. Virol Sin. 2010. PMID: 20960278 Free PMC article. Review. - Regulation of the G2/M transition by p53.
Taylor WR, Stark GR. Taylor WR, et al. Oncogene. 2001 Apr 5;20(15):1803-15. doi: 10.1038/sj.onc.1204252. Oncogene. 2001. PMID: 11313928 Review.
Cited by
- Herpes simplex virus immediate-early protein ICP22 triggers loss of serine 2-phosphorylated RNA polymerase II.
Fraser KA, Rice SA. Fraser KA, et al. J Virol. 2007 May;81(10):5091-101. doi: 10.1128/JVI.00184-07. Epub 2007 Mar 7. J Virol. 2007. PMID: 17344289 Free PMC article. - The products of the herpes simplex virus type 1 immediate-early US1/US1.5 genes downregulate levels of S-phase-specific cyclins and facilitate virus replication in S-phase Vero cells.
Orlando JS, Astor TL, Rundle SA, Schaffer PA. Orlando JS, et al. J Virol. 2006 Apr;80(8):4005-16. doi: 10.1128/JVI.80.8.4005-4016.2006. J Virol. 2006. PMID: 16571817 Free PMC article. - Herpes simplex virus protein kinase US3 activates and functionally overlaps protein kinase A to block apoptosis.
Benetti L, Roizman B. Benetti L, et al. Proc Natl Acad Sci U S A. 2004 Jun 22;101(25):9411-6. doi: 10.1073/pnas.0403160101. Epub 2004 Jun 10. Proc Natl Acad Sci U S A. 2004. PMID: 15192152 Free PMC article. - Role of cellular phosphatase cdc25C in herpes simplex virus 1 replication.
Smith-Donald BA, Durand LO, Roizman B. Smith-Donald BA, et al. J Virol. 2008 May;82(9):4527-32. doi: 10.1128/JVI.02021-07. Epub 2008 Feb 13. J Virol. 2008. PMID: 18272575 Free PMC article. - Distinct and separate roles for herpesvirus-conserved UL97 kinase in cytomegalovirus DNA synthesis and encapsidation.
Wolf DG, Courcelle CT, Prichard MN, Mocarski ES. Wolf DG, et al. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1895-900. doi: 10.1073/pnas.98.4.1895. Proc Natl Acad Sci U S A. 2001. PMID: 11172047 Free PMC article.
References
- Barratt R A, Kao G, McKenna W G, Kuang J, Muschel R J. The G2 block induced by DNA damage: a caffeine-resistant component independent of cdc25C, mpm-2 phosphorylation, and H1 kinase activity. Cancer Res. 1998;58:2639–2645. - PubMed
- Berns J I. Parvoviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Virology. 3rd ed. Vol. 2. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2173–2197.
- Booher R N, Hlman P S, Fattaey A. Human myt1 is a cell cycle-regulated kinase that inhibits cdc2 but not dck2 activity. J Biol Chem. 1997;272:22300–22306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA78766/CA/NCI NIH HHS/United States
- R01 CA078766/CA/NCI NIH HHS/United States
- CA71933/CA/NCI NIH HHS/United States
- CA47451/CA/NCI NIH HHS/United States
- P01 CA071933/CA/NCI NIH HHS/United States
- R37 CA078766/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous