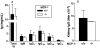Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice - PubMed (original) (raw)
Monocyte chemoattractant protein 1-dependent leukocytic infiltrates are responsible for autoimmune disease in MRL-Fas(lpr) mice
G H Tesch et al. J Exp Med. 1999.
Abstract
Infiltrating leukocytes may be responsible for autoimmune disease. We hypothesized that the chemokine monocyte chemoattractant protein (MCP)-1 recruits macrophages and T cells into tissues that, in turn, are required for autoimmune disease. Using the MRL-Fas(lpr) strain with spontaneous, fatal autoimmune disease, we constructed MCP-1-deficient MRL-Fas(lpr) mice. In MCP-1-intact MRL-Fas(lpr) mice, macrophages and T cells accumulate at sites (kidney tubules, glomeruli, pulmonary bronchioli, lymph nodes) in proportion to MCP-1 expression. Deleting MCP-1 dramatically reduces macrophage and T cell recruitment but not proliferation, protects from kidney, lung, skin, and lymph node pathology, reduces proteinuria, and prolongs survival. Notably, serum immunoglobulin (Ig) isotypes and kidney Ig/C3 deposits are not diminished in MCP-1-deficient MRL-Fas(lpr) mice, highlighting the requirement for MCP-1-dependent leukocyte recruitment to initiate autoimmune disease. However, MCP-1-deficient mice are not completely protected from leukocytic invasion. T cells surrounding vessels with meager MCP-1 expression remain. In addition, downstream effector cytokines/chemokines are decreased in MCP-1-deficient mice, perhaps reflecting a reduction of cytokine-expressing leukocytes. Thus, MCP-1 promotes MRL-Fas(lpr) autoimmune disease through macrophage and T cell recruitment, amplified by increasing local cytokines/chemokines. We suggest that MCP-1 is a principal therapeutic target with which to combat autoimmune diseases.
Figures
Figure 1
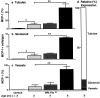
MCP transcripts increase with advancing disease in MRL-Faslpr kidneys. The renal cortex was isolated from C57BL/6 and MRL-Faslpr kidneys from 2 to 6 mo of age and was analyzed for MCP-1, -3, and -5 by reverse transcriptase (RT)-PCR. MCP-1, -3, and -5 are increased in MRL-Faslpr kidneys with progressive disease. Examples of the amplified PCR products are illustrated in the gel photos. Graph: data = mean ± SD; n = 3; *P < 0.01 vs. MRL-Faslpr (2 mo).
Figure 1
MCP transcripts increase with advancing disease in MRL-Faslpr kidneys. The renal cortex was isolated from C57BL/6 and MRL-Faslpr kidneys from 2 to 6 mo of age and was analyzed for MCP-1, -3, and -5 by reverse transcriptase (RT)-PCR. MCP-1, -3, and -5 are increased in MRL-Faslpr kidneys with progressive disease. Examples of the amplified PCR products are illustrated in the gel photos. Graph: data = mean ± SD; n = 3; *P < 0.01 vs. MRL-Faslpr (2 mo).
Figure 2
MCP-1 increases with advancing kidney disease in MRL-Faslpr mice. MCP-1 was assessed by immunostaining in (a) cortical tubules, (b) glomeruli, and (c) vessels in MRL-Faslpr kidneys. Data = mean ± SD; *P < 0.05; **P < 0.005. (d) The relative proportion of MRL-Faslpr kidney cells expressing MCP-1 was similar at 2, 4, and 6 mo of age. Few infiltrating cells in the interstitium (≤1%) express MCP-1.
Figure 3
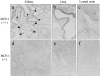
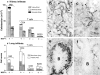
MCP-1 expression in MRL-Faslpr kidney, lung, and lymph nodes. Tissues from MCP-1–intact (a–c) and MCP-1–deficient (e and f) MRL-Faslpr mice at 5 mo of age were immunostained for MCP-1. MCP-1 is strongly expressed by TECs and glomerular podocytes (a) but is weak in vessels (inset) in MCP-1–intact MRL-Faslpr kidneys. Bronchiolar epithelial cells are the main source of MCP-1 in MCP-1–intact MRL-Faslpr lungs (b). A large proportion of infiltrating cells surrounding lymphatics express MCP-1 within the enlarged lymph nodes of MCP-1–intact MRL-Faslpr mice (c). MCP-1 is not detected in MCP-1–deficient kidney, lung, and lymph node (d–f). a and d, ×800; b, c, e, and f, ×500.
Figure 4
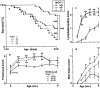
MCP-1–deficient MRL-Faslpr mice are protected from lethal autoimmune injury. (a) We evaluated survival in MCP-1–intact (+/+, +/−) and –deficient (−/−) MRL-Faslpr mice (<50% male and female per group). The survival of MCP-1–intact compared with –deficient MRL-Faslpr mice is markedly reduced (P < 0.0001). In addition, MCP-1+/− MRL-Faslpr mice survive longer than the MCP-1+/+ strain (P < 0.0001). (b) As the collection of small daily volumes of urine is compromised by evaporation problems, we evaluated fresh urine samples using a spot analysis. However, spot analysis in individual samples has several limitations, including small sample volume and semiquantitative measurement. With these caveats in mind, we now report that MCP-1–deficient MRL-Faslpr mice are protected from proteinuria (2–6 mo) in comparison to the MCP-1–intact MRL-Faslpr strain. The number of surviving MCP-1–intact MRL-Faslpr mice declines rapidly after 6 mo of age; therefore, proteinuria at these ages is limited to a subset of MCP-1–intact MRL-Faslpr mice, which are more resistant to disease (normal = B6/129 wild type). (c) Lymphadenopathy is reduced in MCP-1–deficient MRL-Faslpr mice; however, lymphadenopathy is greater in MCP-1–intact MRL-Faslpr females as compared with males (P < 0.01; females in graph). (d) Inflammatory skin lesions are reduced in MCP-1–deficient compared with –intact strains. Data = mean ± SEM; *P < 0.05; **P < 0.005; and ***P < 0.0001 compared with MCP-1−/−.
Figure 5
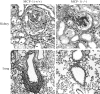
Kidney and lung histopathology are reduced in MCP-1–deficient MRL-Faslpr mice. Tubular damage (arrowheads) and glomerular crescents (arrow) are severe in MCP-1–intact MRL-Faslpr kidneys (a; hematoxylin and PAS) and are reduced in the MCP-1–deficient MRL-Faslpr strain (b) at 5 mo of age. Peribronchial and perivascular cell infiltration in MRL-Faslpr lungs are prominent in the MCP-1 (+/+) strain at 5 mo of age (c). By comparison, the peribronchial infiltrate is reduced in the MCP-1 (−/−) MRL-Faslpr strain (d). a and b, ×800; c and d, ×500.
Figure 6
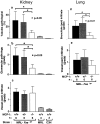
MCP-1–deficient MRL-Faslpr mice are protected from kidney and lung damage during renal disease. MCP-1 intact (+/+, +/−) and deficient (−/−) MRL-Faslpr kidneys were assessed for (a) tubular damage, (b) glomerular damage, and (c) perivascular cell infiltrate at 5 mo of age and compared with age-matched wild-type MRL++ C3H/FeJ strains. MCP-1–intact (+/+, +/−) and deficient (−/−) MRL-Faslpr lungs were analyzed for (d) peribronchiolar and (e) perivascular cell infiltrate at 5 mo of age. Kidney (tubular and glomerular) and lung (bronchiolar) pathology but not vasculitis was reduced in MCP-1–deficient versus MCP-1–intact MRL-Faslpr mice. Data = mean ± SD.
Figure 7
Kidney- and lung-infiltrating leukocytes are reduced in MCP-1–deficient MRL-Faslpr mice. Infiltrating leukocytes were assessed in the kidney (a–c) and lung (d–f) by immunostaining. Macrophage (MØ) accumulation adjacent to kidney parenchymal cells (peritubular, periglomerular, intraglomerular) in MCP-1–intact MRL-Faslpr mice (b) is markedly reduced in MCP-1–deficient MRL-Faslpr mice (c). Similarly, the notable macrophage accumulation adjacent to parenchymal cells in the lung (peribronchiolar) in MCP-1–intact MRL-Faslpr mice (e) is dramatically less in MCP-1–deficient MRL-Faslpr mice (f). Furthermore, T cells, including CD4 and CD8 T cells, are reduced in the peritubular area, whereas CD4 T cells accumulate less surrounding glomeruli in MCP-1–deficient versus MCP-1–intact MRL-Faslpr strains (a). In contrast, perivascular macrophages and T cells are not different in the MCP-1–intact and –deficient MRL-Faslpr lungs (d) and kidneys (not shown). Data = mean ± SD; n = 6; *P < 0.05 and **P < 0.01 compared with MCP-1+/+. F4/80 immunostaining: b and c, ×500; e and f, ×330.
Figure 9
(a) MCP-1 deficiency does not reduce serum Ig isotype levels in MRL-Faslpr mice. Serum from MCP-1–intact and –deficient MRL-Faslpr mice at 5 mo of age was analyzed for Igs by ELISA. (b) MCP-1–deficient MRL-Faslpr mice (5 mo of age) have equivalent amounts of IgG and C3 (not shown) in the kidney despite a reduction in loss of renal function, glomerular and tubular injury, and enhanced survival as compared with the MCP-1–intact strain. Data = mean ± SD; n = 6.
Figure 8
MCP-1 deficiency reduces CSF-1, IFN-γ, and other CCR2 ligands in MRL-Faslpr kidneys. Transcript levels of CSF-1, IFN-γ, MCP-3, and MCP-5 were assessed in comparison to GAPDH in MCP-1–intact and –deficient MRL-Faslpr and B6/129 mouse renal cortices at 5 mo of age by RT-PCR. CSF-1, IFN-γ, MCP-3, and MCP-5 transcripts were reduced in MCP-1–deficient versus MCP-1–intact MRL-Faslpr mice. Data = mean ± SD; n = 6; P values compared with MCP-1–intact MRL-Faslpr mice.
Similar articles
- Negative role of colony-stimulating factor-1 in macrophage, T cell, and B cell mediated autoimmune disease in MRL-Fas(lpr) mice.
Lenda DM, Stanley ER, Kelley VR. Lenda DM, et al. J Immunol. 2004 Oct 1;173(7):4744-54. doi: 10.4049/jimmunol.173.7.4744. J Immunol. 2004. PMID: 15383612 - Chemokines in autoimmune lacrimal gland disease in MRL/MpJ mice.
Akpek EK, Jabs DA, Gérard HC, Prendergast RA, Hudson AP, Lee B, Whittum-Hudson JA. Akpek EK, et al. Invest Ophthalmol Vis Sci. 2004 Jan;45(1):185-90. doi: 10.1167/iovs.03-0812. Invest Ophthalmol Vis Sci. 2004. PMID: 14691172 - IL-12 deficiency in MRL-Fas(lpr) mice delays nephritis and intrarenal IFN-gamma expression, and diminishes systemic pathology.
Kikawada E, Lenda DM, Kelley VR. Kikawada E, et al. J Immunol. 2003 Apr 1;170(7):3915-25. doi: 10.4049/jimmunol.170.7.3915. J Immunol. 2003. PMID: 12646661 - Costimulation by B7-1 and B7-2 is required for autoimmune disease in MRL-Faslpr mice.
Kinoshita K, Tesch G, Schwarting A, Maron R, Sharpe AH, Kelley VR. Kinoshita K, et al. J Immunol. 2000 Jun 1;164(11):6046-56. doi: 10.4049/jimmunol.164.11.6046. J Immunol. 2000. PMID: 10820290 - Genetic basis of autoimmune disease in MRL/lpr mice: dissection of the complex pathological manifestations and their susceptibility loci.
Nose M, Nishihara M, Kamogawa J, Terada M, Nakatsuru S. Nose M, et al. Rev Immunogenet. 2000;2(1):154-64. Rev Immunogenet. 2000. PMID: 11324688 Review.
Cited by
- Maintenance of anti-Sm/RNP autoantibody production by plasma cells residing in ectopic lymphoid tissue and bone marrow memory B cells.
Weinstein JS, Delano MJ, Xu Y, Kelly-Scumpia KM, Nacionales DC, Li Y, Lee PY, Scumpia PO, Yang L, Sobel E, Moldawer LL, Reeves WH. Weinstein JS, et al. J Immunol. 2013 Apr 15;190(8):3916-27. doi: 10.4049/jimmunol.1201880. Epub 2013 Mar 18. J Immunol. 2013. PMID: 23509349 Free PMC article. - Leukocytes in glomerular injury.
Holdsworth SR, Tipping PG. Holdsworth SR, et al. Semin Immunopathol. 2007 Nov;29(4):355-74. doi: 10.1007/s00281-007-0097-9. Epub 2007 Oct 16. Semin Immunopathol. 2007. PMID: 17938927 Review. - Immunohistochemical study of monocyte chemoattractant protein-1 in the pancreas of NOD mice following cyclophosphamide administration and during spontaneous diabetes.
Bai Y, Robinson E, Chai R, Ross JM, Reddy S. Bai Y, et al. J Mol Histol. 2006 May;37(3-4):101-13. doi: 10.1007/s10735-006-9045-6. Epub 2006 Jul 29. J Mol Histol. 2006. PMID: 17063385 - Lupus serum IgG induces skin inflammation through the TNFR1 signaling pathway.
Deng GM, Liu L, Kyttaris VC, Tsokos GC. Deng GM, et al. J Immunol. 2010 Jun 15;184(12):7154-61. doi: 10.4049/jimmunol.0902514. Epub 2010 May 14. J Immunol. 2010. PMID: 20483718 Free PMC article. - Evaluation of the Involvement of Heme Oxygenase-1 Expression in Discoid Lupus Erythematosus Lesions.
Fagone P, Piombino E, Mangano K, De Pasquale R, Nicoletti F, Caltabiano R. Fagone P, et al. Antioxidants (Basel). 2023 Jun 27;12(7):1352. doi: 10.3390/antiox12071352. Antioxidants (Basel). 2023. PMID: 37507892 Free PMC article.
References
- Koh D.R., Ho A., Rahemtulla A., Fung-Leung W.P., Griesser H., Mak T.W. Murine lupus in MRL/lpr mice lacking CD4 or CD8 T cells. Eur. J. Immunol. 1995;25:2558–2562. - PubMed
- Tran E.H., Hoekstra K., van Rooijen N., Dijkstra C.D., Owens T. Immune invasion of the central nervous system parenchyma and experimental allergic encephalomyelitis, but not leukocyte extravasation from blood, are prevented in macrophage-depleted mice. J. Immunol. 1998;161:3767–3775. - PubMed
- Rovin B.H., Rumancik M., Tan L., Dickerson J. Glomerular expression of monocyte chemoattractant protein-1 in experimental and human glomerulonephritis. Lab. Invest. 1994;71:536–542. - PubMed
- Wada T., Yokoyama H., Su S., Mukaida N., Iwano M., Dohi K., Takahashi Y., Sasaki T., Furuichi K., Segawa C. Monitoring urinary levels of monocyte chemotactic and activating factor reflects disease activity of lupus nephritis. Kidney Int. 1996;49:761–767. - PubMed
- Harigai M., Hara M., Yoshimura T., Leonard E.J., Inoue K., Kashiwazaki S. Monocyte chemoattractant protein-1 (MCP-1) in inflammatory joint diseases and its involvement in the cytokine network of rheumatoid synovium. Clin. Immunol. Immunopathol. 1993;69:83–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- Z01 DK036149/ImNIH/Intramural NIH HHS/United States
- DK 52369/DK/NIDDK NIH HHS/United States
- R01 DK052369/DK/NIDDK NIH HHS/United States
- R01 DK036149/DK/NIDDK NIH HHS/United States
- DK-36149/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous