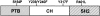Bifurcation of cell migratory and proliferative signaling by the adaptor protein Shc - PubMed (original) (raw)
Bifurcation of cell migratory and proliferative signaling by the adaptor protein Shc
L R Collins et al. J Cell Biol. 1999.
Abstract
Cytokines and extracellular matrix proteins initiate signaling cascades that regulate cell migration and proliferation. Evidence is provided that the adaptor protein Shc can differentially regulate these processes. Specifically, under growth factor-limiting conditions, Shc stimulates haptotactic cell migration without affecting anchorage-dependent proliferation. However, when growth factors are present, Shc no longer influences cell migration; rather, Shc is crucial for DNA synthesis. Mutational analysis of Shc demonstrates that, while tyrosine phosphorylation is required for both DNA synthesis and cell migration, the switch in Shc signaling is associated with differential use of Shc's phosphotyrosine interacting domains; the PTB domain regulates haptotaxis, while the SH2 domain is selectively required for proliferation.
Figures
Figure 1
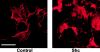
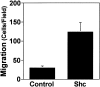
Decreased actin organization and increased migration of Shc expressing Cos-7 cells. (Left) Serum-deprived control and Shc stably expressing cells were harvested, plated on collagen-coated coverslips for 2 h, fixed, permeabilized, and stained with rhodamine phalloidin. The images shown are single confocal optical sections taken with a 63× objective (left). The scale bar is equal to 50 μm. (Right) Migration of parental vs Shc-expressing Cos-7 cells. Serum-deprived cells were allowed to migrate for 4 h in Boyden chambers towards a collagen matrix. Data from one of four independent experiments are shown (mean ± SE). The enhanced migration of the Shc cell line was found to be statistically significant (P < 0.05 by Student's t test). Random migration towards BSA-coated chambers was always <2% and subtracted from each value.
Figure 1
Decreased actin organization and increased migration of Shc expressing Cos-7 cells. (Left) Serum-deprived control and Shc stably expressing cells were harvested, plated on collagen-coated coverslips for 2 h, fixed, permeabilized, and stained with rhodamine phalloidin. The images shown are single confocal optical sections taken with a 63× objective (left). The scale bar is equal to 50 μm. (Right) Migration of parental vs Shc-expressing Cos-7 cells. Serum-deprived cells were allowed to migrate for 4 h in Boyden chambers towards a collagen matrix. Data from one of four independent experiments are shown (mean ± SE). The enhanced migration of the Shc cell line was found to be statistically significant (P < 0.05 by Student's t test). Random migration towards BSA-coated chambers was always <2% and subtracted from each value.
Figure 2
Shc stimulates migration on collagen and vitronectin. (Top) Cos-7 cells were either mock transfected (pcDNA3.1HisC plus lac z) or transfected with p52 Shc cDNA plus lac z. Cells were deprived of serum and migration was assessed as indicated with either migration media (mock, p52) or 100 ng/ml EGF (Mock+EGF) in the lower chamber. Migration was quantitated by enumerating the number of β-galactosidase positive cells/field using a 20× objective. The mean ± SE from a representative of five independent experiments is shown. (Bottom) Tyrosine phosphorylation of p52 Shc in response to integrin ligation. Serum-deprived cells expressing p52 Shc were harvested, then either held in suspension or replated on collagen or vitronectin for 1 h and lysed in RIPA buffer. His-tagged Shc was isolated with nickel agarose beads and analyzed for phosphotyrosine content by Western blot (mAb 4G10, upper autoradiogram). The blot was then stripped and reprobed with an anti-Shc antibody as described in Materials and Methods (lower autoradiogram).
Figure 3
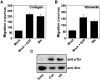
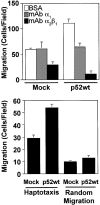
Shc stimulates haptotactic migration. (Top) Shc-stimulated cell migration is integrin dependent. Cells were processed for migration assay as described in Materials and Methods, except that cells were mixed with anti-integrin monoclonal antibodies as indicated before loading into Boyden chambers. Next, cells were allowed to migrate for 4 h and quantitated for migration as described above. Each bar represents the mean ± SE from one experiment representative of three with similar results. (Bottom) Serum-deprived mock or p52 Shc transfected Cos-7 cells were allowed to migrate on Boyden chambers coated on either their lower surface (haptotaxis) or both their upper and lower surfaces (random migration). Each bar represents the mean ± SE of a representative experiment performed in triplicate.
Figure 4
Schematic of Shc constructs. Full-length p52 Shc is depicted. Gray, white, and hatched regions represent the NH2-terminal PTB domain, the CH domain, and the SH2 domain respectively. The point mutations used are indicated above the schematic. Mutants are identified by S154P, Y239/Y240F, Y317F, Y239/Y240/Y317F (Shc 3YF), and R401L.
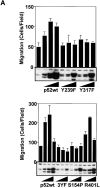
Figure 5
Mutational analysis of Shc-stimulated migration. (A) Cos-7 cells were transfected with increasing amounts of the indicated Shc cDNAs (0.1, 0.3, and 3.0 μg/10-cm plate), serum deprived and assessed for haptotaxis towards collagen. Each bar represents the mean ± SE of a representative experiment. Lysates were generated from the cells used in these experiments and analyzed by immunoblotting with polyclonal anti-Shc antibodies. His-tagged Shc can be discerned from endogenous Shc in the Western blot by its slightly retarded mobility on the gel. The 0.3 μg/plate condition was repeated at least five times for all the mutants with similar results. Cell migration induced by both p52wt and R401L was found to be highly statistically significant (P < 0.01 by ANOVA). (B) tyrosine phosphorylation pattern of Shc mutants. Cells were either held in suspension or plated on collagen or vitronectin. As a positive control, cells were stimulated with insulin as indicated. His-tagged Shc was isolated after lysis in RIPA buffer by incubation with nickel agarose beads and analyzed by Western blotting with anti-phosphotyrosine. Blots were then stripped and reprobed with pAb Shc.
Figure 5
Mutational analysis of Shc-stimulated migration. (A) Cos-7 cells were transfected with increasing amounts of the indicated Shc cDNAs (0.1, 0.3, and 3.0 μg/10-cm plate), serum deprived and assessed for haptotaxis towards collagen. Each bar represents the mean ± SE of a representative experiment. Lysates were generated from the cells used in these experiments and analyzed by immunoblotting with polyclonal anti-Shc antibodies. His-tagged Shc can be discerned from endogenous Shc in the Western blot by its slightly retarded mobility on the gel. The 0.3 μg/plate condition was repeated at least five times for all the mutants with similar results. Cell migration induced by both p52wt and R401L was found to be highly statistically significant (P < 0.01 by ANOVA). (B) tyrosine phosphorylation pattern of Shc mutants. Cells were either held in suspension or plated on collagen or vitronectin. As a positive control, cells were stimulated with insulin as indicated. His-tagged Shc was isolated after lysis in RIPA buffer by incubation with nickel agarose beads and analyzed by Western blotting with anti-phosphotyrosine. Blots were then stripped and reprobed with pAb Shc.
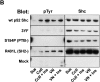
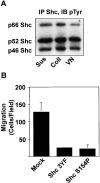
Figure 6
Shc requirement for haptotaxis in a metastatic cell line. (A) Shc is constitutively phosphorylated in human FG-M pancreatic carcinoma cells. Serum-deprived FG-M cells were either held in suspension or plated on extracellular matrix proteins as indicated for 30 min. Cells were then lysed and analyzed by immunoblotting with anti-phosphotyrosine as described in Materials and Methods. (B) FG-M cells require Shc tyrosine phosphorylation and the Shc PTB domain for haptotaxis. FG-M cells were transfected as indicated and assayed for haptotaxis on vitronectin by counting the number of transfected cells/high powered field. Each bar represents the mean ± SE from a single representative experiment performed in triplicate.
Figure 7
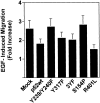
Shc is not required for EGF-stimulated cell migration. Cos-7 cells were transfected with cDNAs encoding Shc mutants as indicated, serum deprived, and assayed for migration in the presence of 100 ng/ml EGF as described in Materials and Methods. The data are depicted as relative migration, with the migration induced by the indicated forms of Shc in the absence of EGF defined as one. Each bar represents the mean ± SE of four independent experiments performed in duplicate. None of the differences between the groups were found to be statistically significant.
Figure 8
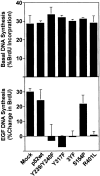
Shc is required for EGF to stimulate anchorage-dependent DNA synthesis. Cos-7 cells were transfected with the indicated cDNAs, serum deprived and assayed for BrdU incorporation in either the absence (top) or the presence of 100 ng/ml EGF (bottom). The data are presented as the percentage of transfected cells incorporating BrdU (top) or as percent increase in BrdU incorporation stimulated by the addition of EGF (bottom). Each bar represents the mean ± SD of three independent experiments performed in triplicate.
Similar articles
- Tyrosine phosphorylation-dependent and -independent role of Shc in the regulation of IGF-1-induced mitogenesis and glycogen synthesis.
Sasaoka T, Ishiki M, Wada T, Hori H, Hirai H, Haruta T, Ishihara H, Kobayashi M. Sasaoka T, et al. Endocrinology. 2001 Dec;142(12):5226-35. doi: 10.1210/endo.142.12.8543. Endocrinology. 2001. PMID: 11713219 - Neuregulin-increased expression of acetylcholine receptor epsilon-subunit gene requires ErbB interaction with Shc.
Won S, Si J, Colledge M, Ravichandran KS, Froehner SC, Mei L. Won S, et al. J Neurochem. 1999 Dec;73(6):2358-68. doi: 10.1046/j.1471-4159.1999.0732358.x. J Neurochem. 1999. PMID: 10582594 - Shc phosphotyrosine-binding domain dominantly interacts with epidermal growth factor receptors and mediates Ras activation in intact cells.
Sakaguchi K, Okabayashi Y, Kido Y, Kimura S, Matsumura Y, Inushima K, Kasuga M. Sakaguchi K, et al. Mol Endocrinol. 1998 Apr;12(4):536-43. doi: 10.1210/mend.12.4.0094. Mol Endocrinol. 1998. PMID: 9544989 - The PI/PTB domain: a new protein interaction domain involved in growth factor receptor signaling.
Margolis B. Margolis B. J Lab Clin Med. 1996 Sep;128(3):235-41. doi: 10.1016/s0022-2143(96)90022-0. J Lab Clin Med. 1996. PMID: 8783629 Review. - [A variety of functions of conventional and neuronal Shc adapter molecules in phosphotyrosine-mediated signalings].
Nakamura T, Mori N. Nakamura T, et al. Seikagaku. 2001 Nov;73(11):1322-5. Seikagaku. 2001. PMID: 11831027 Review. Japanese. No abstract available.
Cited by
- EphB1 recruits c-Src and p52Shc to activate MAPK/ERK and promote chemotaxis.
Vindis C, Cerretti DP, Daniel TO, Huynh-Do U. Vindis C, et al. J Cell Biol. 2003 Aug 18;162(4):661-71. doi: 10.1083/jcb.200302073. J Cell Biol. 2003. PMID: 12925710 Free PMC article. - Opioid System and Epithelial-Mesenchymal Transition.
Łazarczyk M, Skiba D, Mickael ME, Jaskuła K, Nawrocka A, Religa P, Sacharczuk M. Łazarczyk M, et al. Pharmaceuticals (Basel). 2025 Jan 17;18(1):120. doi: 10.3390/ph18010120. Pharmaceuticals (Basel). 2025. PMID: 39861181 Free PMC article. Review. - The SHCA adapter protein cooperates with lipoma-preferred partner in the regulation of adhesion dynamics and invadopodia formation.
Kiepas A, Voorand E, Senecal J, Ahn R, Annis MG, Jacquet K, Tali G, Bisson N, Ursini-Siegel J, Siegel PM, Brown CM. Kiepas A, et al. J Biol Chem. 2020 Jul 31;295(31):10535-10559. doi: 10.1074/jbc.RA119.011903. Epub 2020 Apr 16. J Biol Chem. 2020. PMID: 32299913 Free PMC article. - A novel role of Shc adaptor proteins in steroid hormone-regulated cancers.
Alam SM, Rajendran M, Ouyang S, Veeramani S, Zhang L, Lin MF. Alam SM, et al. Endocr Relat Cancer. 2009 Mar;16(1):1-16. doi: 10.1677/ERC-08-0179. Epub 2008 Nov 11. Endocr Relat Cancer. 2009. PMID: 19001530 Free PMC article. Review. - Mechanism of Akt1 inhibition of breast cancer cell invasion reveals a protumorigenic role for TSC2.
Liu H, Radisky DC, Nelson CM, Zhang H, Fata JE, Roth RA, Bissell MJ. Liu H, et al. Proc Natl Acad Sci U S A. 2006 Mar 14;103(11):4134-9. doi: 10.1073/pnas.0511342103. Epub 2006 Mar 7. Proc Natl Acad Sci U S A. 2006. PMID: 16537497 Free PMC article.
References
- Anand-Apte B., Zetter B.R., Viswanathan A., Qiu R.G., Chen J., Ruggieri R., Symons M. Platelet-derived growth factor and fibronectin-stimulated migration are differentially regulated by the Rac and extracellular signal-regulated kinase pathways. J. Biol. Chem. 1997;272:30688–30692. - PubMed
- Blaikie P., Immanuel D., Wu J., Li N., Yajnik V., Margolis B. A region in Shc distinct from the SH2 domain can bind tyrosine-phosphorylated growth factor receptors. J. Biol. Chem. 1994;269:32031–32034. - PubMed
- Bonfini L., Migliaccio E., Pelicci G., Lanfrancone L., Pelicci P.G. Not all Shc's roads lead to Ras. Trends Biochem. Sci. 1996;21:257–261. - PubMed
- Cowley S., Paterson H., Kemp P., Marshall C.J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. - PubMed
- Gotoh N., Muroya K., Hattori S., Nakamura S., Chida K., Shibuya M. The SH2 domain of Shc suppresses EGF-induced mitogenesis in a dominant negative manner. Oncogene. 1995;11:2525–2533. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL54444/HL/NHLBI NIH HHS/United States
- R37 CA050286/CA/NCI NIH HHS/United States
- P01 CA078045/CA/NCI NIH HHS/United States
- R01 CA045726/CA/NCI NIH HHS/United States
- CA45726/CA/NCI NIH HHS/United States
- R01 CA050286/CA/NCI NIH HHS/United States
- CA50286/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous