CD40 ligation induces tissue factor expression in human vascular smooth muscle cells - PubMed (original) (raw)
CD40 ligation induces tissue factor expression in human vascular smooth muscle cells
U Schönbeck et al. Am J Pathol. 2000 Jan.
Abstract
Tissue factor (TF) instigates the extrinsic pathway of blood coagulation. Plaque disruption and exposure of circulating factor VII/VIIa to subendothelial procoagulants such as TF leads to intravascular thrombosis, a frequent cause of acute atherosclerotic events. Although the expression of TF in the intima of human atherosclerotic lesions is well established, little is known about the mechanisms of TF regulation in vascular smooth muscle cells (SMC). We demonstrate here that TF colocalizes with the receptor CD40 on lesional SMC within atherosclerotic lesions in situ. In cultured vascular SMC, ligation of CD40 with native CD40 ligand (CD40L) derived from activated T lymphocytes or recombinant human CD40L (rCD40L) induced the transient expression of TF on the cell surface (as determined by FACS analysis) in a concentration- and time-dependent manner and enhanced total cell-associated TF (as determined by ELISA). CD40L-induced TF on vascular SMC is functional and activates coagulation. In accordance with the increased TF activity, stimulation of vascular SMC with rCD40L did not affect either protein expression or activity of tissue factor pathway inhibitors. In summary, these findings demonstrate the potential of the CD40/CD40L signaling pathway to augment the procoagulant activity in human vascular SMC. Because TF and CD40 colocalize on lesional SMC in human atheroma, CD40/CD40L signaling may contribute to the TF expression and hence to increased thrombogenicity of plaques during the inflammatory responses of atherogenesis and arterial injury.
Figures
Figure 1.
Tissue factor colocalizes with CD40-positive smooth muscle cells in human atherosclerotic lesions. Frozen sections of human carotid lesions were analyzed for α-actin (top panels: left, ×100; right, ×400), CD40 (left middle panel, green, ×400) and TF (lower left panel, red, ×400) staining within human atherosclerotic lesions, employing immunhistochemistry (for SMC) and immunofluorescence double labeling (for CD40 and TF). The lumen of the artery is at the top of each photomicrograph. Staining employing control IgG is shown for CD40 (right middle panel, ×400) and TF (right lower panel, ×400). Analysis of atheroma from six different donors showed similar results.
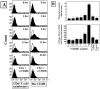
Figure 2.
CD40L induces tissue factor in human vascular smooth muscle cells in vitro. A: FACS analysis for human TF on vascular SMC, cultured 24 hours before the experiment in IT medium and subsequently for the indicated times with cell-membrane preparations of PMA-activated (50 ng/ml, 12 hours) T lymphocytes (left panels) or recombinant CD40L (10 μg/ml rCD40L; right panels) in the absence or presence of the α-CD40L mAb (α-CD40L, 10 μg/ml). Staining performed with control IgG is shown as lines. B: Lysates of human vascular SMC, previously cultured 24 hours in IT medium, then incubated for 24 hours with the respective concentrations of recombinant human CD40 ligand (rCD40L) in the absence or presence of the α-CD40L mAb (α-CD40L, 10 μg/ml), were analyzed for TF protein expression by ELISA (upper panel). TF activity was analyzed in membrane-preparations of human vascular SMC (lower panel), as described in Materials and Methods. Data shown are representative of three experiments performed with cells of different origins.
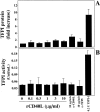
Figure 3.
CD40L does not affect expression of TFPI in human vascular SMC. Human vascular SMC, cultured 24 hours before the experiment in IT medium, were incubated for 24 hours with the respective concentrations of rCD40L in the absence or presence of the anti-CD40L mAb (α-CD40L, 10 μg/ml) and analyzed for TFPI expression by ELISA (A) as well as TFPI-activity assay (B). Recombinant TFPI-1 was applied for control purposes. Data shown are representative of three and seven experiments, respectively, performed with cells of different origins.
Similar articles
- Functional CD40 ligand is expressed on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for CD40-CD40 ligand signaling in atherosclerosis.
Mach F, Schönbeck U, Sukhova GK, Bourcier T, Bonnefoy JY, Pober JS, Libby P. Mach F, et al. Proc Natl Acad Sci U S A. 1997 Mar 4;94(5):1931-6. doi: 10.1073/pnas.94.5.1931. Proc Natl Acad Sci U S A. 1997. PMID: 9050882 Free PMC article. - Expression of stromelysin-3 in atherosclerotic lesions: regulation via CD40-CD40 ligand signaling in vitro and in vivo.
Schönbeck U, Mach F, Sukhova GK, Atkinson E, Levesque E, Herman M, Graber P, Basset P, Libby P. Schönbeck U, et al. J Exp Med. 1999 Mar 1;189(5):843-53. doi: 10.1084/jem.189.5.843. J Exp Med. 1999. PMID: 10049948 Free PMC article. - CD40 ligand (CD40L) does not stimulate proliferation of vascular smooth muscle cells.
Hermann A, Schrör K, Weber AA. Hermann A, et al. Eur J Cell Biol. 2002 Apr;81(4):213-21. doi: 10.1078/0171-9335-00240. Eur J Cell Biol. 2002. PMID: 12018389 - CD40 signaling in vascular cells: a key role in atherosclerosis?
Mach F, Schönbeck U, Libby P. Mach F, et al. Atherosclerosis. 1998 Apr;137 Suppl:S89-95. doi: 10.1016/s0021-9150(97)00309-2. Atherosclerosis. 1998. PMID: 9694547 Review. - CD40 signaling and plaque instability.
Schönbeck U, Libby P. Schönbeck U, et al. Circ Res. 2001 Dec 7;89(12):1092-103. doi: 10.1161/hh2401.101272. Circ Res. 2001. PMID: 11739273 Review.
Cited by
- Renewal of mural thrombus releases plasma markers and is involved in aortic abdominal aneurysm evolution.
Touat Z, Ollivier V, Dai J, Huisse MG, Bezeaud A, Sebbag U, Palombi T, Rossignol P, Meilhac O, Guillin MC, Michel JB. Touat Z, et al. Am J Pathol. 2006 Mar;168(3):1022-30. doi: 10.2353/ajpath.2006.050868. Am J Pathol. 2006. PMID: 16507915 Free PMC article. - Both early and delayed anti-CD40L antibody treatment induces a stable plaque phenotype.
Lutgens E, Cleutjens KB, Heeneman S, Koteliansky VE, Burkly LC, Daemen MJ. Lutgens E, et al. Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):7464-9. doi: 10.1073/pnas.97.13.7464. Proc Natl Acad Sci U S A. 2000. PMID: 10861013 Free PMC article. - Beyond thrombosis: the impact of tissue factor signaling in cancer.
Unruh D, Horbinski C. Unruh D, et al. J Hematol Oncol. 2020 Jul 14;13(1):93. doi: 10.1186/s13045-020-00932-z. J Hematol Oncol. 2020. PMID: 32665005 Free PMC article. Review. - Effect of cyclosporin A intervention on the immunological mechanisms of coronary heart disease and restenosis.
Wang X, Hu YC, Zhang RY, Jin DX, Jiang Y, Zhang HN, Cong HL. Wang X, et al. Exp Ther Med. 2016 Nov;12(5):3242-3248. doi: 10.3892/etm.2016.3775. Epub 2016 Oct 4. Exp Ther Med. 2016. PMID: 27882144 Free PMC article. - Crucial role of CD40 signaling in vascular wall cells in neointimal formation and vascular remodeling after vascular interventions.
Song Z, Jin R, Yu S, Nanda A, Granger DN, Li G. Song Z, et al. Arterioscler Thromb Vasc Biol. 2012 Jan;32(1):50-64. doi: 10.1161/ATVBAHA.111.238329. Epub 2011 Oct 13. Arterioscler Thromb Vasc Biol. 2012. PMID: 21998133 Free PMC article.
References
- Fuster V, Badimon I, Badimon JJ, Chesebro JH: The pathogenesis of coronary artery disease and the acute coronary syndromes. N Engl J Med 1992, 326:242-250 - PubMed
- Nemerson Y: Tissue factor and hemostasis. Blood 1988, 71:1-8 - PubMed
- Edgington TS, Mackman N, Brand K, Ruf W: The structutal biology of expression and function of tissue factor. Thromb Haemost 1991, 66:67-79 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous