Lineage-restricted function of nuclear factor kappaB-inducing kinase (NIK) in transducing signals via CD40 - PubMed (original) (raw)
Lineage-restricted function of nuclear factor kappaB-inducing kinase (NIK) in transducing signals via CD40
N Garceau et al. J Exp Med. 2000.
Abstract
CD40 signaling in B cells and dendritic cells (DCs) is critical for the development of humoral and cell-mediated immunity, respectively. Nuclear factor kappaB (NF-kappaB)-inducing kinase (NIK) has been implicated as a central transducing kinase in CD40-dependent activation. Here, we show that although NIK is essential for B cell activation, it is dispensable for activation of DCs. Such data provide compelling evidence that different intermediary kinases are used by different cellular lineages to trigger NF-kappaB activation via CD40.
Figures
Figure 1
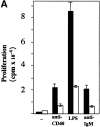
Role of NIK in activation of B cells. aly/aly and aly/+ splenic B cells were cultured in vitro and assayed for their ability to proliferate (A), produce Ig (B), and upregulate cell surface markers (C) in response to CD40 stimulation. (A) Induction of proliferation by anti-CD40 (10 μg/ml), FGK115, LPS (50 μg/ml), and anti-IgM (goat anti–mouse IgM) was measured by the incorporation of [3H]thymidine from 66 to 72 h after initiation of culture. All cultures contained 10 ng/ml of IL-4. (B) Induction of Ig secretion was performed using sCD154 or LPS in combination with IL-4 (10 ng/ml) and IL-5 (10 ng/ml). Ig secretion was measured using an isotype-specific ELISA. (C) Expression of cell surface molecules on splenic B cells from _aly/_+ (top) or aly/aly (bottom) mice after culture with (purple histogram) or without (green outline histogram) sCD154 for 48 h was measured by flow cytometry.
Figure 1
Role of NIK in activation of B cells. aly/aly and aly/+ splenic B cells were cultured in vitro and assayed for their ability to proliferate (A), produce Ig (B), and upregulate cell surface markers (C) in response to CD40 stimulation. (A) Induction of proliferation by anti-CD40 (10 μg/ml), FGK115, LPS (50 μg/ml), and anti-IgM (goat anti–mouse IgM) was measured by the incorporation of [3H]thymidine from 66 to 72 h after initiation of culture. All cultures contained 10 ng/ml of IL-4. (B) Induction of Ig secretion was performed using sCD154 or LPS in combination with IL-4 (10 ng/ml) and IL-5 (10 ng/ml). Ig secretion was measured using an isotype-specific ELISA. (C) Expression of cell surface molecules on splenic B cells from _aly/_+ (top) or aly/aly (bottom) mice after culture with (purple histogram) or without (green outline histogram) sCD154 for 48 h was measured by flow cytometry.
Figure 1
Role of NIK in activation of B cells. aly/aly and aly/+ splenic B cells were cultured in vitro and assayed for their ability to proliferate (A), produce Ig (B), and upregulate cell surface markers (C) in response to CD40 stimulation. (A) Induction of proliferation by anti-CD40 (10 μg/ml), FGK115, LPS (50 μg/ml), and anti-IgM (goat anti–mouse IgM) was measured by the incorporation of [3H]thymidine from 66 to 72 h after initiation of culture. All cultures contained 10 ng/ml of IL-4. (B) Induction of Ig secretion was performed using sCD154 or LPS in combination with IL-4 (10 ng/ml) and IL-5 (10 ng/ml). Ig secretion was measured using an isotype-specific ELISA. (C) Expression of cell surface molecules on splenic B cells from _aly/_+ (top) or aly/aly (bottom) mice after culture with (purple histogram) or without (green outline histogram) sCD154 for 48 h was measured by flow cytometry.
Figure 3
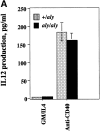
Responses of DCs in aly/aly and aly/+ mice. (A) The NIK mutation does not affect CD40-induced IL-12 production by DCs. _aly/_+ or aly/aly DCs were cultured at 2 × 106 cells/ml in cRPMI with GM-CSF/IL-4 (both at 10 ng/ml) with or without anti-CD40 (10 μg/ml). Culture supernatant was assayed for IL-12 on day 3 by ELISA. (B) The NIK mutation does not affect the antigen-presentation capacity of DCs. DCs were purified as in A and were pulsed with OVA peptide (323–339) for 90 min, washed extensively, and then plated with 105 OTII cells (OVA-specific Tg T cells) at various DC densities as indicated. At 48 h, culture supernatants were assayed for the presence of IL-2 by ELISA.
Figure 3
Responses of DCs in aly/aly and aly/+ mice. (A) The NIK mutation does not affect CD40-induced IL-12 production by DCs. _aly/_+ or aly/aly DCs were cultured at 2 × 106 cells/ml in cRPMI with GM-CSF/IL-4 (both at 10 ng/ml) with or without anti-CD40 (10 μg/ml). Culture supernatant was assayed for IL-12 on day 3 by ELISA. (B) The NIK mutation does not affect the antigen-presentation capacity of DCs. DCs were purified as in A and were pulsed with OVA peptide (323–339) for 90 min, washed extensively, and then plated with 105 OTII cells (OVA-specific Tg T cells) at various DC densities as indicated. At 48 h, culture supernatants were assayed for the presence of IL-2 by ELISA.
Figure 2
Activation of NF-κB by NIK and aly NIK. Effect of aly NIK expression on IκBα phosphorylation in primary B cells. B cells from aly/aly and aly/+ mice were stimulated in vitro for 2, 5, or 15 min with sCD154, or for 5 min with LPS (50 μg/ml) or PMA (10 ng/ml) plus ionomycin (25 nM), and were assayed for phosphorylation of IκBα by Western blot with a phospho-specific anti-IκBα.
Figure 4
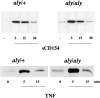
Phosphorylation of IκBα in response to CD40 engagement is normal in DCs from aly/aly mice. DCs were stimulated in vitro with sCD154 or TNF-α as indicated, and were assayed for phosphorylation of IκBα by Western blot with a phospho-specific IκBα antibody.
Similar articles
- Non-canonical NF-κB signaling pathway.
Sun SC. Sun SC. Cell Res. 2011 Jan;21(1):71-85. doi: 10.1038/cr.2010.177. Epub 2010 Dec 21. Cell Res. 2011. PMID: 21173796 Free PMC article. Review. - Roles of TRAF6 in CD40 signaling.
Hostager BS. Hostager BS. Immunol Res. 2007;39(1-3):105-14. doi: 10.1007/s12026-007-0082-3. Immunol Res. 2007. PMID: 17917059 Review.
Cited by
- The IkappaB function of NF-kappaB2 p100 controls stimulated osteoclastogenesis.
Novack DV, Yin L, Hagen-Stapleton A, Schreiber RD, Goeddel DV, Ross FP, Teitelbaum SL. Novack DV, et al. J Exp Med. 2003 Sep 1;198(5):771-81. doi: 10.1084/jem.20030116. Epub 2003 Aug 25. J Exp Med. 2003. PMID: 12939342 Free PMC article. - CD40 regulates the processing of NF-kappaB2 p100 to p52.
Coope HJ, Atkinson PG, Huhse B, Belich M, Janzen J, Holman MJ, Klaus GG, Johnston LH, Ley SC. Coope HJ, et al. EMBO J. 2002 Oct 15;21(20):5375-85. doi: 10.1093/emboj/cdf542. EMBO J. 2002. PMID: 12374738 Free PMC article. - NF-κB inducing kinase: a key regulator in the immune system and in cancer.
Thu YM, Richmond A. Thu YM, et al. Cytokine Growth Factor Rev. 2010 Aug;21(4):213-26. doi: 10.1016/j.cytogfr.2010.06.002. Epub 2010 Aug 3. Cytokine Growth Factor Rev. 2010. PMID: 20685151 Free PMC article. Review. - Structure of the nuclear factor κB-inducing kinase (NIK) kinase domain reveals a constitutively active conformation.
Liu J, Sudom A, Min X, Cao Z, Gao X, Ayres M, Lee F, Cao P, Johnstone S, Plotnikova O, Walker N, Chen G, Wang Z. Liu J, et al. J Biol Chem. 2012 Aug 10;287(33):27326-34. doi: 10.1074/jbc.M112.366658. Epub 2012 Jun 20. J Biol Chem. 2012. PMID: 22718757 Free PMC article. - CD40 signaling in human dendritic cells is initiated within membrane rafts.
Vidalain PO, Azocar O, Servet-Delprat C, Rabourdin-Combe C, Gerlier D, Manié S. Vidalain PO, et al. EMBO J. 2000 Jul 3;19(13):3304-13. doi: 10.1093/emboj/19.13.3304. EMBO J. 2000. PMID: 10880443 Free PMC article.
References
- Noelle R.J. CD40 and its ligand in cell-mediated immunity. Agents Actions Suppl. 1998;49:17–22. - PubMed
- Xu J., Foy T.M., Laman J.D., Elliott E.A., Dunn J.J., Waldschmidt T.J., Elsemore J., Noelle R.J., Flavell R.A. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. - PubMed
- Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. The immune responses in CD40-deficient miceimpaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. - PubMed
- Foy T.M., Aruffo A., Ledbetter J.A., Noelle R.J. In vivo CD40–gp39 interactions are essential for thymus-dependent immunity. II. Prolonged in vivo suppression of primary and secondary humoral immune responses by an antibody targeted to the CD40 ligand, gp39. J. Exp. Med. 1993;178:1567–1575. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous