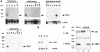FHL2, a novel tissue-specific coactivator of the androgen receptor - PubMed (original) (raw)
FHL2, a novel tissue-specific coactivator of the androgen receptor
J M Müller et al. EMBO J. 2000.
Abstract
The control of target gene expression by nuclear receptors requires the recruitment of multiple cofactors. However, the exact mechanisms by which nuclear receptor-cofactor interactions result in tissue-specific gene regulation are unclear. Here we characterize a novel tissue-specific coactivator for the androgen receptor (AR), which is identical to a previously reported protein FHL2/DRAL with unknown function. In the adult, FHL2 is expressed in the myocardium of the heart and in the epithelial cells of the prostate, where it colocalizes with the AR in the nucleus. FHL2 contains a strong, autonomous transactivation function and binds specifically to the AR in vitro and in vivo. In an agonist- and AF-2-dependent manner FHL2 selectively increases the transcriptional activity of the AR, but not that of any other nuclear receptor. In addition, the transcription of the prostate-specific AR target gene probasin is coactivated by FHL2. Taken together, our data demonstrate that FHL2 is the first LIM-only coactivator of the AR with a unique tissue-specific expression pattern.
Figures
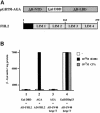
Fig. 1. FHL2 interacts with the AR in yeast in an agonist-dependent manner. (A) Schematic representation of the AGA bait protein and the FHL2 protein. (B) β-galactosidase activity in yeast expressing AGA and Gal4AD–FHL2 in the presence or absence of the agonist R1881 or the antagonist CPA (bar 2). Bars 1 and 3 show the negative and bar 4 shows the positive controls, respectively.
Fig. 2. Analysis of FHL2 expression. (A) FHL2 is specifically expressed in the heart. Northern blots of human tissues (fetal, adult and adult muscle) and adult mouse tissues were probed with FHL2. β–actin was used as an internal control. (B) FHL2 is mainly expressed in the heart ventricles. Northern blot analysis of mRNA of left and right ventricles (LV, RV) and left and right atria (LA, RA) of an explanted human heart. GAPDH was used as a control. (C) FHL2 and AR protein expression in human heart. Extract (200 μg) from a left ventricle was analysed in a Western blot. Controls are shown in lane 2 (10 μg of 293 cell extract transfected with human AR) and lane 3 (100 ng of purified His-tagged FHL2). The upper panel was decorated with an α–AR antibody, the lower panel with an α–FHL2-specific antibody. (D) Immunohistochemical staining of FHL2 in cardiac muscle cells (a and b) or FHL2 and AR in prostate epithelium (c–f). Controls are shown in b, d and f. In the heart, FHL2 antibodies stained specifically myocardial fibres (my), but not vascular smooth muscle cells (sm) (a). Two hematoxilin–eosin stained arteriolae (at) shown in (a) entirely lack FHL2 immunoreactivity. Both cytoplasmic and nuclear FHL2 immunoreactivity was detected in the secretory epithelium of the prostate (c). AR immunoreactivity was confined to the nucleus of the secretory epithelium (e). Abbreviations: at, arteriola; br, brain; co, colon; ep, epithelial cells; ht, heart; kd, kidney; li, liver; lm, lumen; lu, lung; my, myofibres; pan, pancreas; pl, placenta; pr, prostate; skm, skeletal muscle; si, small intestine; sm, smooth muscle; sp, spleen; st, stomach; str, stroma; te, testis; ut, uterus.
Fig. 2. Analysis of FHL2 expression. (A) FHL2 is specifically expressed in the heart. Northern blots of human tissues (fetal, adult and adult muscle) and adult mouse tissues were probed with FHL2. β–actin was used as an internal control. (B) FHL2 is mainly expressed in the heart ventricles. Northern blot analysis of mRNA of left and right ventricles (LV, RV) and left and right atria (LA, RA) of an explanted human heart. GAPDH was used as a control. (C) FHL2 and AR protein expression in human heart. Extract (200 μg) from a left ventricle was analysed in a Western blot. Controls are shown in lane 2 (10 μg of 293 cell extract transfected with human AR) and lane 3 (100 ng of purified His-tagged FHL2). The upper panel was decorated with an α–AR antibody, the lower panel with an α–FHL2-specific antibody. (D) Immunohistochemical staining of FHL2 in cardiac muscle cells (a and b) or FHL2 and AR in prostate epithelium (c–f). Controls are shown in b, d and f. In the heart, FHL2 antibodies stained specifically myocardial fibres (my), but not vascular smooth muscle cells (sm) (a). Two hematoxilin–eosin stained arteriolae (at) shown in (a) entirely lack FHL2 immunoreactivity. Both cytoplasmic and nuclear FHL2 immunoreactivity was detected in the secretory epithelium of the prostate (c). AR immunoreactivity was confined to the nucleus of the secretory epithelium (e). Abbreviations: at, arteriola; br, brain; co, colon; ep, epithelial cells; ht, heart; kd, kidney; li, liver; lm, lumen; lu, lung; my, myofibres; pan, pancreas; pl, placenta; pr, prostate; skm, skeletal muscle; si, small intestine; sm, smooth muscle; sp, spleen; st, stomach; str, stroma; te, testis; ut, uterus.
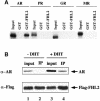
Fig. 3. FHL2 interacts with the AR in vitro and in vivo. (A) Selective interaction of FHL2 with the AR. GST pulldown assays were performed with in vitro translated, labelled AR, PR, GR or MR in the presence of their cognate ligands and GST–FHL2 fusion protein. GST protein was used as a control. (B) AR coimmunoprecipitates with FHL2 in the presence of the natural agonist DHT. Nuclear extracts of 293 cells transfected with AR and Flag-FHL2 were immunoprecipitated with α–Flag antibody. Ten percent of the extract used for immunoprecipitation was loaded as input in lanes 1 and 3. The immunoprecipitate (IP) is loaded in lanes 2 and 4. Western blots were either decorated with an α–Flag- or an α–AR-specific antibody.
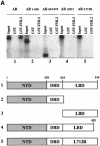
Fig. 4. AR associates with FHL2 in an AF-2-dependent manner. (A) GST pulldown assays were performed using GST–FHL2 fusion protein and in vitro translated, labelled AR or AR mutants in the presence of the agonist R1881. (B) Interaction between the AR and either GST, GST–FHL2, GST–FHL2(1–162) or GST–FHL2(163–279) fusion proteins in the presence of the agonist R1881. Numbers above the scheme indicate the amino acid position.
Fig. 4. AR associates with FHL2 in an AF-2-dependent manner. (A) GST pulldown assays were performed using GST–FHL2 fusion protein and in vitro translated, labelled AR or AR mutants in the presence of the agonist R1881. (B) Interaction between the AR and either GST, GST–FHL2, GST–FHL2(1–162) or GST–FHL2(163–279) fusion proteins in the presence of the agonist R1881. Numbers above the scheme indicate the amino acid position.
Fig. 5. FHL2 contains an autonomous transactivation function. The Gal–FHL2 fusion protein transactivates the G5E1b-LUC reporter gene in different cell lines (CV-1, 293 and HL-1). The coactivators Gal–TIF2.1 and Gal–SRC1a were used as controls.
Fig. 6. FHL2 is a specific coactivator of the AR. (A) FHL2 coactivates the AR in an agonist- and AF-2-dependent manner. Expression plasmids coding for AR, ARΔH12, PR, GR or MR were cotransfected with the MMTV-LUC reporter with or without FHL2 in 293 cells. (B) FHL2, TIF2, SRC1e or SRC1a coactivate the AR to a similar extent in 293 cells. (C) FHL2 functions as a coactivator of the AR-specific probasin-LUC reporter (PB-LUC). AR and FHL2 were cotransfected as indicated in the presence of two different AR agonists (R1881, DHT) or the antagonist CPA in CV-1 cells.
Fig. 6. FHL2 is a specific coactivator of the AR. (A) FHL2 coactivates the AR in an agonist- and AF-2-dependent manner. Expression plasmids coding for AR, ARΔH12, PR, GR or MR were cotransfected with the MMTV-LUC reporter with or without FHL2 in 293 cells. (B) FHL2, TIF2, SRC1e or SRC1a coactivate the AR to a similar extent in 293 cells. (C) FHL2 functions as a coactivator of the AR-specific probasin-LUC reporter (PB-LUC). AR and FHL2 were cotransfected as indicated in the presence of two different AR agonists (R1881, DHT) or the antagonist CPA in CV-1 cells.
Fig. 6. FHL2 is a specific coactivator of the AR. (A) FHL2 coactivates the AR in an agonist- and AF-2-dependent manner. Expression plasmids coding for AR, ARΔH12, PR, GR or MR were cotransfected with the MMTV-LUC reporter with or without FHL2 in 293 cells. (B) FHL2, TIF2, SRC1e or SRC1a coactivate the AR to a similar extent in 293 cells. (C) FHL2 functions as a coactivator of the AR-specific probasin-LUC reporter (PB-LUC). AR and FHL2 were cotransfected as indicated in the presence of two different AR agonists (R1881, DHT) or the antagonist CPA in CV-1 cells.
Similar articles
- Regulation of the transcriptional coactivator FHL2 licenses activation of the androgen receptor in castrate-resistant prostate cancer.
McGrath MJ, Binge LC, Sriratana A, Wang H, Robinson PA, Pook D, Fedele CG, Brown S, Dyson JM, Cottle DL, Cowling BS, Niranjan B, Risbridger GP, Mitchell CA. McGrath MJ, et al. Cancer Res. 2013 Aug 15;73(16):5066-79. doi: 10.1158/0008-5472.CAN-12-4520. Epub 2013 Jun 25. Cancer Res. 2013. PMID: 23801747 - Proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor enhances androgen receptor functions through LIM-only coactivator, four-and-a-half LIM-only protein 2.
Nair SS, Guo Z, Mueller JM, Koochekpour S, Qiu Y, Tekmal RR, Schüle R, Kung HJ, Kumar R, Vadlamudi RK. Nair SS, et al. Mol Endocrinol. 2007 Mar;21(3):613-24. doi: 10.1210/me.2006-0269. Epub 2006 Dec 27. Mol Endocrinol. 2007. PMID: 17192406 Free PMC article. - Androgen receptor coactivators lysine-specific histone demethylase 1 and four and a half LIM domain protein 2 predict risk of prostate cancer recurrence.
Kahl P, Gullotti L, Heukamp LC, Wolf S, Friedrichs N, Vorreuther R, Solleder G, Bastian PJ, Ellinger J, Metzger E, Schüle R, Buettner R. Kahl P, et al. Cancer Res. 2006 Dec 1;66(23):11341-7. doi: 10.1158/0008-5472.CAN-06-1570. Cancer Res. 2006. PMID: 17145880 - Expression and function of androgen receptor coactivators in prostate cancer.
Culig Z, Comuzzi B, Steiner H, Bartsch G, Hobisch A. Culig Z, et al. J Steroid Biochem Mol Biol. 2004 Nov;92(4):265-71. doi: 10.1016/j.jsbmb.2004.10.003. Epub 2004 Dec 19. J Steroid Biochem Mol Biol. 2004. PMID: 15663989 Review. - The biological relevance of FHL2 in tumour cells and its role as a putative cancer target.
Kleiber K, Strebhardt K, Martin BT. Kleiber K, et al. Anticancer Res. 2007 Jan-Feb;27(1A):55-61. Anticancer Res. 2007. PMID: 17352216 Review.
Cited by
- A genome-wide RNA interference screen identifies new regulators of androgen receptor function in prostate cancer cells.
Imberg-Kazdan K, Ha S, Greenfield A, Poultney CS, Bonneau R, Logan SK, Garabedian MJ. Imberg-Kazdan K, et al. Genome Res. 2013 Apr;23(4):581-91. doi: 10.1101/gr.144774.112. Epub 2013 Feb 12. Genome Res. 2013. PMID: 23403032 Free PMC article. - Deficiency in the LIM-only protein Fhl2 impairs skin wound healing.
Wixler V, Hirner S, Müller JM, Gullotti L, Will C, Kirfel J, Günther T, Schneider H, Bosserhoff A, Schorle H, Park J, Schüle R, Buettner R. Wixler V, et al. J Cell Biol. 2007 Apr 9;177(1):163-72. doi: 10.1083/jcb.200606043. J Cell Biol. 2007. PMID: 17420295 Free PMC article. - Differential ligand-dependent protein-protein interactions between nuclear receptors and a neuronal-specific cofactor.
Greiner EF, Kirfel J, Greschik H, Huang D, Becker P, Kapfhammer JP, Schüle R. Greiner EF, et al. Proc Natl Acad Sci U S A. 2000 Jun 20;97(13):7160-5. doi: 10.1073/pnas.97.13.7160. Proc Natl Acad Sci U S A. 2000. PMID: 10860982 Free PMC article. - From SNP co-association to RNA co-expression: novel insights into gene networks for intramuscular fatty acid composition in porcine.
Ramayo-Caldas Y, Ballester M, Fortes MR, Esteve-Codina A, Castelló A, Noguera JL, Fernández AI, Pérez-Enciso M, Reverter A, Folch JM. Ramayo-Caldas Y, et al. BMC Genomics. 2014 Mar 26;15:232. doi: 10.1186/1471-2164-15-232. BMC Genomics. 2014. PMID: 24666776 Free PMC article. - Deleted in breast cancer 1, a novel androgen receptor (AR) coactivator that promotes AR DNA-binding activity.
Fu J, Jiang J, Li J, Wang S, Shi G, Feng Q, White E, Qin J, Wong J. Fu J, et al. J Biol Chem. 2009 Mar 13;284(11):6832-40. doi: 10.1074/jbc.M808988200. Epub 2009 Jan 5. J Biol Chem. 2009. PMID: 19126541 Free PMC article.
References
- Alen P., Claessens, F., Schoenmakers, E., Swinnen, J.V., Verhoeven, G., Rombauts, W. and Peeters, B. (1999) Interaction of the putative androgen receptor-specific coactivator ARA70/ELE1α with multiple steroid receptors and identification of an internally deleted ELE1β isoform. Mol. Endocrinol., 13, 117–128. - PubMed
- Arber S., Halder, G. and Caroni, P. (1994) Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell, 79, 221–231. - PubMed
- Arriza J.L., Weinberger, C., Cerelli, G., Glaser, T.M., Handelin, B.L., Housman, D.E. and Evans, R.M. (1987) Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science, 237, 268–275. - PubMed
- Aumüller G., Holterhus, P.M., Konrad, L., von Rahden, B., Hiort, O., Esquenet, M. and Verhoeven, G. (1998) Immunohistochemistry and in situ hybridization of the androgen receptor in the developing human prostate. Anat. Embryol. (Berl.), 197, 199–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials