A phosphotyrosine displacement mechanism for activation of Src by PTPalpha - PubMed (original) (raw)
A phosphotyrosine displacement mechanism for activation of Src by PTPalpha
X M Zheng et al. EMBO J. 2000.
Abstract
Protein tyrosine phosphatase alpha (PTPalpha) is believed to dephosphorylate physiologically the Src proto-oncogene at phosphotyrosine (pTyr)527, a critical negative-regulatory residue. It thereby activates Src, and PTPalpha overexpression neoplastically transforms NIH 3T3 cells. pTyr789 in PTPalpha is constitutively phosphorylated and binds Grb2, an interaction that may inhibit PTPalpha activity. We show here that this phosphorylation also specifically enables PTPalpha to dephosphorylate pTyr527. Tyr789-->Phe mutation abrogates PTPalpha-Src binding, dephosphorylation of pTyr527 (although not of other substrates), and neoplastic transformation by overexpressed PTPalpha in vivo. We suggest that pTyr789 enables pTyr527 dephosphorylation by a pilot binding with the Src SH2 domain that displaces the intramolecular pTyr527-SH2 binding. Consistent with model predictions, we find that excess SH2 domains can disrupt PTPalpha-Src binding and can block PTPalpha-mediated dephosphorylation and activation in proportion to their affinity for pTyr789. Moreover, we show that, as predicted by the model, catalytically defective PTPalpha has reduced Src binding in vivo. The displacement mechanism provides another potential control point for physiological regulation of Src-family signal transduction pathways.
Figures
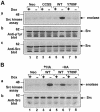
Fig. 1. PTPα inducible expression and subcellular localization. Cell line names are explained in Table I. (A) Lysates containing 20 μg of total cell protein were prepared from control cells (lanes 1 and 2) and from HA-tagged wt (lanes 5 and 6) and mutant (lanes 3–4 and 7–8) PTPα overexpressor cells that had been grown in the absence (induction of expression, even-numbered lanes) or presence (suppression of expression, odd-numbered lanes) of 5 ng/ml doxycycline (Dox). Lysates were immunoblotted with either anti-PTPα polyclonal (panel a) or anti-HA monoclonal (panel b) antibodies. (B) Membrane (M) and cytosolic (C) subcellular fractions from induced cells were purified, and aliquots containing 25 μg of total protein were immunoblotted with anti-PTPα polyclonal antibody 7–091. (C) As in (A), except that lysates were immunoblotted with anti-pTyr antibody 4G10. The positions of molecular weight markers (in kDa) are indicated.
Fig. 1. PTPα inducible expression and subcellular localization. Cell line names are explained in Table I. (A) Lysates containing 20 μg of total cell protein were prepared from control cells (lanes 1 and 2) and from HA-tagged wt (lanes 5 and 6) and mutant (lanes 3–4 and 7–8) PTPα overexpressor cells that had been grown in the absence (induction of expression, even-numbered lanes) or presence (suppression of expression, odd-numbered lanes) of 5 ng/ml doxycycline (Dox). Lysates were immunoblotted with either anti-PTPα polyclonal (panel a) or anti-HA monoclonal (panel b) antibodies. (B) Membrane (M) and cytosolic (C) subcellular fractions from induced cells were purified, and aliquots containing 25 μg of total protein were immunoblotted with anti-PTPα polyclonal antibody 7–091. (C) As in (A), except that lysates were immunoblotted with anti-pTyr antibody 4G10. The positions of molecular weight markers (in kDa) are indicated.
Fig. 1. PTPα inducible expression and subcellular localization. Cell line names are explained in Table I. (A) Lysates containing 20 μg of total cell protein were prepared from control cells (lanes 1 and 2) and from HA-tagged wt (lanes 5 and 6) and mutant (lanes 3–4 and 7–8) PTPα overexpressor cells that had been grown in the absence (induction of expression, even-numbered lanes) or presence (suppression of expression, odd-numbered lanes) of 5 ng/ml doxycycline (Dox). Lysates were immunoblotted with either anti-PTPα polyclonal (panel a) or anti-HA monoclonal (panel b) antibodies. (B) Membrane (M) and cytosolic (C) subcellular fractions from induced cells were purified, and aliquots containing 25 μg of total protein were immunoblotted with anti-PTPα polyclonal antibody 7–091. (C) As in (A), except that lysates were immunoblotted with anti-pTyr antibody 4G10. The positions of molecular weight markers (in kDa) are indicated.
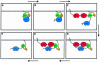
Fig. 2. Colony formation in soft agarose by PTPα overexpressor cells. Control (Neo), HA-tagged (+HA) and -untagged (-HA) wt and Tyr789→Phe mutant PTPα-expressor cells, and a previously characterized v-_src_-transformed cell line (NIH[pMvsrc/foc/ep]A1; Kmiecik and Shalloway, 1997) were cultured in suspension in media containing 0.3% agarose and no doxycycline. Colonies were photographed after 14 days. Cells that overexpressed PTPα(CCSS) formed no colonies and looked identical to the Neo cells (not shown). Similar results were obtained in four independent experiments.
Fig. 3. Effect of PTPα overexpression on Src in vivo tyrosine phosphorylation and kinase activity. Cell line names are explained in Table I. (A) Src was immunoprecipitated from lysates containing 1 mg of total cell protein prepared from control cells (lanes 1 and 2), wt PTPα (lanes 5 and 6) and mutant PTPα (lanes 3–4 and 7–8) expressor cells that had been grown in the presence (odd-numbered lanes) or absence (even-numbered lanes) of doxycycline (Dox). Each immunoprecipitate was divided into three fractions (containing 10, 45 and 45%, respectively, of the total), which were either: (a) subjected to kinase assay in buffer containing [γ-32P]ATP and acid-denatured enolase followed by 9% SDS–PAGE and autoradiography of the reaction products; (b) immunoblotted using anti-pTyr mAb 4G10; or (c) immunoblotted using anti-Src mAb 327. The positions of molecular weight markers (in kDa) are indicated. (B) As in (A) except that Src was immunoprecipitated from lysates from cells overexpressing untagged (–HA) wt PTPα and PTPα(Y789F) (lanes 5–8). Epitope-tagged (+HA) wt PTPα (lanes 3 and 4) was included as a positive control.
Fig. 4. In vitro dephosphorylation and activation of Src by PTPα. Eluates containing anti-HA immunopurified wt and mutant PTPα and equivalent volumes of mock-purified eluates were prepared from induced (–Dox) and uninduced (+Dox) PTPα overexpressor and control cells. (A) Five percent of each eluate was pre-incubated in dephosphorylation buffer with wt Src (bound to GammaBind Sepharose beads) that had been immunoprecipitated from Src overexpressor cells. The Src was then washed and subjected to in vitro kinase assay with [γ-32P]ATP and acid-denatured enolase as in Figure 3. (B) Twenty percent of each eluate was immunoblotted with anti-pTyr antibody. (C) Twenty percent of each eluate was immunoblotted with anti-PTPα antibody. The positions of molecular weight markers (in kDa) are indicated.
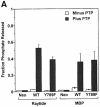
Fig. 5. In vitro dephosphorylation of non-specific and specific substrates by wt and mutant PTPα. (A) Raytide peptide and MBP were tyrosine phosphorylated in vitro using v-Src and [γ-32P]ATP. The radioactive substrates (Raytide: 3.5 × 105 c.p.m./lane, 2 μg/lane; MBP: 1.5 × 105 c.p.m./lane, 6 μg/lane) were then incubated in phosphatase buffer with anti-HA immunoprecipitates from lysates (containing 500 μg of total cell protein) prepared from uninduced and induced control and PTPα-expressor cells for 30 min (i.e. within the linear reaction/time regime) at 30°C. Reactions were terminated and the amount of [32P]phosphate released was measured by scintillation counting. Results are averages of two independent experiments; ranges are indicated. (B) Src was immunoprecipitated from either Src(Y527F) (panels a and b) or wt Src (panels c and d) overexpressor cells (see Table I for full names) and subjected to in vitro dephosphorylation with wt or mutant PTPα purified from induced overexpressor cells (lanes 4, 6 and 8). Mock-purified eluates from non-overexpressor cells (lanes 1 and 2) and uninduced PTPα expressor cells (lanes 3, 5 and 7) were used as controls. Reactions were terminated and divided into two equal aliquots, which were immunoblotted for phosphotyrosine content using anti-pTyr mAb 4G10 (panels a and c) or for Src levels using anti-Src mAb 327 (panels b and d). The positions of molecular weight markers (in kDa) are indicated.
Fig. 5. In vitro dephosphorylation of non-specific and specific substrates by wt and mutant PTPα. (A) Raytide peptide and MBP were tyrosine phosphorylated in vitro using v-Src and [γ-32P]ATP. The radioactive substrates (Raytide: 3.5 × 105 c.p.m./lane, 2 μg/lane; MBP: 1.5 × 105 c.p.m./lane, 6 μg/lane) were then incubated in phosphatase buffer with anti-HA immunoprecipitates from lysates (containing 500 μg of total cell protein) prepared from uninduced and induced control and PTPα-expressor cells for 30 min (i.e. within the linear reaction/time regime) at 30°C. Reactions were terminated and the amount of [32P]phosphate released was measured by scintillation counting. Results are averages of two independent experiments; ranges are indicated. (B) Src was immunoprecipitated from either Src(Y527F) (panels a and b) or wt Src (panels c and d) overexpressor cells (see Table I for full names) and subjected to in vitro dephosphorylation with wt or mutant PTPα purified from induced overexpressor cells (lanes 4, 6 and 8). Mock-purified eluates from non-overexpressor cells (lanes 1 and 2) and uninduced PTPα expressor cells (lanes 3, 5 and 7) were used as controls. Reactions were terminated and divided into two equal aliquots, which were immunoblotted for phosphotyrosine content using anti-pTyr mAb 4G10 (panels a and c) or for Src levels using anti-Src mAb 327 (panels b and d). The positions of molecular weight markers (in kDa) are indicated.
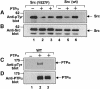
Fig. 6. Effect of dephosphorylation of PTPα on its dephosphorylating activity. (A and B) Src was immunoprecipitated from either Src(Y527F) (lanes 1–3) or wt Src overexpressor cells (lanes 4–6) and incubated with (anti-HA) immunopurified wt PTPα (lanes 2 and 5), dephosphorylated immunopurified wt PTPα (lanes 3 and 6) or mock-purified eluates from uninduced PTPα overexpressor cells (lanes 1 and 4) as in Figure 5. Reactions were terminated and divided into two equal aliquots, which were immunoblotted with either anti-pTyr mAb 4G10 (A) or anti-Src mAb 327 (B). (The band in lane 2 was unevenly blotted because of a bubble.) (C and D) Aliquots of the purified (or mock-purified) PTPα used in the Src dephosphorylation reactions were immunoblotted with either anti-pTyr mAb 4G10 (C) or with anti-PTPα 7-091 polyclonal antibody (D). Lanes 1–3 in these panels correspond to lanes 1–3 and lanes 4–6 in the panels above. The positions of molecular weight markers (in kDa) are indicated.
Fig. 7. Phosphotyrosine displacement model for PTPα substrate specificity. Src pTyr527 is usually bound to the Src SH2 domain (A), but this binding is transiently dissociated by thermal fluctuations (B), providing an opportunity for PTPα pTyr789 binding instead (C). The transient binding provides an extended time period for rearrangement to a head-to-tail conformation (D) that will facilitate Tyr527 dephosphorylation by the D1 catalytic domain. Because there is no further competition from pTyr527, the pTyr789–SH2 binding will persist for a longer time, enhancing the association between PTPα and Src (E), prior to dissociation (F).
Fig. 8. GST–Src SH2 fusion protein inhibits the in vivo association of Src with PTPα. (A) Endogenous Src was immunoprecipitated from lysates (1.5 mg of protein) prepared from the indicated induced HA-tagged PTPα expressor cell lines using anti-Src polyclonal antibody. Equal aliquots of each immunoprecipitate were immunoblotted with either anti-PTPα antibody 7-091 (panel a) or anti-Src mAb 327 (panel b). Immunoblots of whole-cell lysates prior to immunoprecipitation (30 μg of total cell protein) are shown in lanes 1–4. (B) In vivo association of wt PTPα with Src was assayed as described above except that endogenous Src was immunoprecipitated with anti-Src mAb 2-17, and the washed immunoprecipitates were either incubated alone (lane 2) or in the presence of the indicated amounts of purified GST (lanes 3 and 4) or GST–Src SH2 (lanes 5 and 6) fusion proteins for an additional 30 min at 4°C and rewashed prior to immunoblotting. All lanes are with lysates from wt PTPα overexpressor cells. Immunoblots of whole-cell lysate prior to immunoprecipitation (30 μg of protein) are shown in lane 1.
Fig. 8. GST–Src SH2 fusion protein inhibits the in vivo association of Src with PTPα. (A) Endogenous Src was immunoprecipitated from lysates (1.5 mg of protein) prepared from the indicated induced HA-tagged PTPα expressor cell lines using anti-Src polyclonal antibody. Equal aliquots of each immunoprecipitate were immunoblotted with either anti-PTPα antibody 7-091 (panel a) or anti-Src mAb 327 (panel b). Immunoblots of whole-cell lysates prior to immunoprecipitation (30 μg of total cell protein) are shown in lanes 1–4. (B) In vivo association of wt PTPα with Src was assayed as described above except that endogenous Src was immunoprecipitated with anti-Src mAb 2-17, and the washed immunoprecipitates were either incubated alone (lane 2) or in the presence of the indicated amounts of purified GST (lanes 3 and 4) or GST–Src SH2 (lanes 5 and 6) fusion proteins for an additional 30 min at 4°C and rewashed prior to immunoblotting. All lanes are with lysates from wt PTPα overexpressor cells. Immunoblots of whole-cell lysate prior to immunoprecipitation (30 μg of protein) are shown in lane 1.
Fig. 9. GST–Src SH2 fusion protein inhibits the activation of Src by wt PTPα in vitro. Eluates containing (anti-HA) immunopurified wt and mutant PTPα (or equivalent volumes of mock-purified eluates) were prepared from induced (+PTPα; lanes 3, 4, 7 and 8) and uninduced (–PTPα; lanes 1, 2, 5 and 6) wt PTPα (lanes 1–4) or PTPα(Y789F) (lanes 5–8) overexpressor cells, and were pre-incubated without (odd-numbered lanes) or with (even-numbered lanes) 5 μg of GST–Src SH2 fusion protein for 30 min at 4°C. The ability of 10% of each mixture to stimulate Src kinase activity was then tested as described in Figure 4A. The position of a molecular weight marker (in kDa) is indicated.
Fig. 10. Differential inhibition of PTPα-mediated Src activation by Src and Grb2 SH2 domains. (A) Affinity-precipitation of wt PTPα and PTPα(Y789F) by the Src and Grb2 SH2 domains. Fifteen micrograms of either GST (lanes 1, 5 and 9), GST–Src SH2 (lanes 2, 6 and 10) or GST–Grb2 SH2 (lanes 3, 7 and 11) purified fusion protein were incubated without (lanes 1–3) or with (lanes 4–11) lysates (containing 1.5 mg of total cell protein) prepared from wt PTPα (lanes 4–7) or PTPα(Y789F) (lanes 8–11) induced overexpressor cells. Complexes were adsorbed on glutathione beads, and the proteins eluted from the washed beads were analyzed by immunoblotting for PTPα. Lanes 4 and 8 are anti-PTPα immunoblots of whole-cell lysates (30 μg of protein). The position of a molecular weight marker (in kDa) is indicated. (B) Affinity precipitation of wt Src and Src(W148R/Y416F) by the Src and Grb2 SH2 domains. As in (A) except that the GST fusion proteins were incubated with lysates (300 μg of protein) from wt Src overexpressor cells (lanes 2–4) or Src(W148R/Y416F) overexpressor cells (lanes 6–8), and the proteins eluted from the washed glutathione beads were immunoblotted with anti-Src antibody. Lanes 1 and 5 are anti-Src immunoblots of whole-cell lysates (5 μg of protein). (C) Anti-HA immunopurified wt PTPα from induced (+PTPα; lanes 2–10) PTPα overexpressor cells or an equivalent volume of mock-purified eluate from uninduced PTPα overexpressor cells (–PTPα; lane 1) was pre-incubated without (lanes 1 and 2) or with the indicated concentrations of either GST–Grb2 SH2 (lanes 3–7) or GST–Src SH2 (lanes 8–12) fusion proteins for 30 min at 4°C. (Note that the GST–Src SH2 concentrations are 10 times the corresponding GST–Grb2 SH2 concentrations.) Ten percent of each mixture was used with immunoprecipitated wt Src for in vitro dephosphorylation and Src kinase assays as in Figure 9.
Fig. 10. Differential inhibition of PTPα-mediated Src activation by Src and Grb2 SH2 domains. (A) Affinity-precipitation of wt PTPα and PTPα(Y789F) by the Src and Grb2 SH2 domains. Fifteen micrograms of either GST (lanes 1, 5 and 9), GST–Src SH2 (lanes 2, 6 and 10) or GST–Grb2 SH2 (lanes 3, 7 and 11) purified fusion protein were incubated without (lanes 1–3) or with (lanes 4–11) lysates (containing 1.5 mg of total cell protein) prepared from wt PTPα (lanes 4–7) or PTPα(Y789F) (lanes 8–11) induced overexpressor cells. Complexes were adsorbed on glutathione beads, and the proteins eluted from the washed beads were analyzed by immunoblotting for PTPα. Lanes 4 and 8 are anti-PTPα immunoblots of whole-cell lysates (30 μg of protein). The position of a molecular weight marker (in kDa) is indicated. (B) Affinity precipitation of wt Src and Src(W148R/Y416F) by the Src and Grb2 SH2 domains. As in (A) except that the GST fusion proteins were incubated with lysates (300 μg of protein) from wt Src overexpressor cells (lanes 2–4) or Src(W148R/Y416F) overexpressor cells (lanes 6–8), and the proteins eluted from the washed glutathione beads were immunoblotted with anti-Src antibody. Lanes 1 and 5 are anti-Src immunoblots of whole-cell lysates (5 μg of protein). (C) Anti-HA immunopurified wt PTPα from induced (+PTPα; lanes 2–10) PTPα overexpressor cells or an equivalent volume of mock-purified eluate from uninduced PTPα overexpressor cells (–PTPα; lane 1) was pre-incubated without (lanes 1 and 2) or with the indicated concentrations of either GST–Grb2 SH2 (lanes 3–7) or GST–Src SH2 (lanes 8–12) fusion proteins for 30 min at 4°C. (Note that the GST–Src SH2 concentrations are 10 times the corresponding GST–Grb2 SH2 concentrations.) Ten percent of each mixture was used with immunoprecipitated wt Src for in vitro dephosphorylation and Src kinase assays as in Figure 9.
Fig. 10. Differential inhibition of PTPα-mediated Src activation by Src and Grb2 SH2 domains. (A) Affinity-precipitation of wt PTPα and PTPα(Y789F) by the Src and Grb2 SH2 domains. Fifteen micrograms of either GST (lanes 1, 5 and 9), GST–Src SH2 (lanes 2, 6 and 10) or GST–Grb2 SH2 (lanes 3, 7 and 11) purified fusion protein were incubated without (lanes 1–3) or with (lanes 4–11) lysates (containing 1.5 mg of total cell protein) prepared from wt PTPα (lanes 4–7) or PTPα(Y789F) (lanes 8–11) induced overexpressor cells. Complexes were adsorbed on glutathione beads, and the proteins eluted from the washed beads were analyzed by immunoblotting for PTPα. Lanes 4 and 8 are anti-PTPα immunoblots of whole-cell lysates (30 μg of protein). The position of a molecular weight marker (in kDa) is indicated. (B) Affinity precipitation of wt Src and Src(W148R/Y416F) by the Src and Grb2 SH2 domains. As in (A) except that the GST fusion proteins were incubated with lysates (300 μg of protein) from wt Src overexpressor cells (lanes 2–4) or Src(W148R/Y416F) overexpressor cells (lanes 6–8), and the proteins eluted from the washed glutathione beads were immunoblotted with anti-Src antibody. Lanes 1 and 5 are anti-Src immunoblots of whole-cell lysates (5 μg of protein). (C) Anti-HA immunopurified wt PTPα from induced (+PTPα; lanes 2–10) PTPα overexpressor cells or an equivalent volume of mock-purified eluate from uninduced PTPα overexpressor cells (–PTPα; lane 1) was pre-incubated without (lanes 1 and 2) or with the indicated concentrations of either GST–Grb2 SH2 (lanes 3–7) or GST–Src SH2 (lanes 8–12) fusion proteins for 30 min at 4°C. (Note that the GST–Src SH2 concentrations are 10 times the corresponding GST–Grb2 SH2 concentrations.) Ten percent of each mixture was used with immunoprecipitated wt Src for in vitro dephosphorylation and Src kinase assays as in Figure 9.
Similar articles
- Mitotic activation of protein-tyrosine phosphatase alpha and regulation of its Src-mediated transforming activity by its sites of protein kinase C phosphorylation.
Zheng XM, Resnick RJ, Shalloway D. Zheng XM, et al. J Biol Chem. 2002 Jun 14;277(24):21922-9. doi: 10.1074/jbc.M201394200. Epub 2002 Mar 28. J Biol Chem. 2002. PMID: 11923305 Free PMC article. - Two mechanisms activate PTPalpha during mitosis.
Zheng XM, Shalloway D. Zheng XM, et al. EMBO J. 2001 Nov 1;20(21):6037-49. doi: 10.1093/emboj/20.21.6037. EMBO J. 2001. PMID: 11689444 Free PMC article. - Grb2 promotes integrin-induced focal adhesion kinase (FAK) autophosphorylation and directs the phosphorylation of protein tyrosine phosphatase α by the Src-FAK kinase complex.
Cheng SY, Sun G, Schlaepfer DD, Pallen CJ. Cheng SY, et al. Mol Cell Biol. 2014 Feb;34(3):348-61. doi: 10.1128/MCB.00825-13. Epub 2013 Nov 18. Mol Cell Biol. 2014. PMID: 24248601 Free PMC article. - Protein tyrosine phosphatase alpha (PTPalpha): a Src family kinase activator and mediator of multiple biological effects.
Pallen CJ. Pallen CJ. Curr Top Med Chem. 2003;3(7):821-35. doi: 10.2174/1568026033452320. Curr Top Med Chem. 2003. PMID: 12678847 Review. - Src kinase regulation by phosphorylation and dephosphorylation.
Roskoski R Jr. Roskoski R Jr. Biochem Biophys Res Commun. 2005 May 27;331(1):1-14. doi: 10.1016/j.bbrc.2005.03.012. Biochem Biophys Res Commun. 2005. PMID: 15845350 Review.
Cited by
- Comprehensive binary interaction mapping of SH2 domains via fluorescence polarization reveals novel functional diversification of ErbB receptors.
Hause RJ Jr, Leung KK, Barkinge JL, Ciaccio MF, Chuu CP, Jones RB. Hause RJ Jr, et al. PLoS One. 2012;7(9):e44471. doi: 10.1371/journal.pone.0044471. Epub 2012 Sep 4. PLoS One. 2012. PMID: 22973453 Free PMC article. - S-nitrosylation of c-Src via NMDAR-nNOS module promotes c-Src activation and NR2A phosphorylation in cerebral ischemia/reperfusion.
Tang LJ, Li C, Hu SQ, Wu YP, Zong YY, Sun CC, Zhang F, Zhang GY. Tang LJ, et al. Mol Cell Biochem. 2012 Jun;365(1-2):363-77. doi: 10.1007/s11010-012-1280-4. Epub 2012 Mar 16. Mol Cell Biochem. 2012. PMID: 22422045 - Protein tyrosine phosphatase α phosphotyrosyl-789 binds BCAR3 to position Cas for activation at integrin-mediated focal adhesions.
Sun G, Cheng SY, Chen M, Lim CJ, Pallen CJ. Sun G, et al. Mol Cell Biol. 2012 Sep;32(18):3776-89. doi: 10.1128/MCB.00214-12. Epub 2012 Jul 16. Mol Cell Biol. 2012. PMID: 22801373 Free PMC article. - A TRIF-independent branch of TLR3 signaling.
Yamashita M, Chattopadhyay S, Fensterl V, Zhang Y, Sen GC. Yamashita M, et al. J Immunol. 2012 Mar 15;188(6):2825-33. doi: 10.4049/jimmunol.1103220. Epub 2012 Feb 8. J Immunol. 2012. PMID: 22323545 Free PMC article. - RPTP-alpha acts as a transducer of mechanical force on alphav/beta3-integrin-cytoskeleton linkages.
von Wichert G, Jiang G, Kostic A, De Vos K, Sap J, Sheetz MP. von Wichert G, et al. J Cell Biol. 2003 Apr 14;161(1):143-53. doi: 10.1083/jcb.200211061. Epub 2003 Apr 7. J Cell Biol. 2003. PMID: 12682088 Free PMC article.
References
- Alema S., Cassalbore, P., Agostini, E. and Tato, F. (1985) Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature, 316, 557–559. - PubMed
- Bagrodia S., Chackalaparampil, I., Kmiecik, T.E. and Shalloway, D. (1991) Altered tyrosine 527 phosphorylation and mitotic activation of p60c-src. Nature, 349, 172–175. - PubMed
- Bagrodia S., Laudano, A.P. and Shalloway, D. (1994) Accessibility of the c-src SH2-domain for binding is increased during mitosis. J. Biol. Chem., 269, 10247–10251. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous