Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses - PubMed (original) (raw)
Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses
H Kissel et al. EMBO J. 2000.
Abstract
The Kit receptor tyrosine kinase functions in hemato- poiesis, melanogenesis and gametogenesis. Kit receptor-mediated cellular responses include proliferation, survival, adhesion, secretion and differentiation. In mast cells, Kit-mediated recruitment and activation of phosphatidylinositol 3'-kinase (PI 3-kinase) produces phosphatidylinositol 3'-phosphates, plays a critical role in mediating cell adhesion and secretion and has contributory roles in mediating cell survival and proliferation. To investigate the consequences in vivo of blocking Kit-mediated PI 3-kinase activation we have mutated the binding site for the p85 subunit of PI 3-kinase in the Kit gene, using a knock-in strategy. Mutant mice have no pigment deficiency or impairment of steady-state hematopoiesis. However, gametogenesis is affected in several ways and tissue mast cell numbers are affected differentially. While primordial germ cells during embryonic development are not affected, Kit(Y719F)/Kit(Y719F) males are sterile due to a block at the premeiotic stages in spermatogenesis. Furthermore, adult males develop Leydig cell hyperplasia. The Leydig cell hyperplasia implies a role for Kit in Leydig cell differentiation and/or steroidogenesis. In mutant females follicle development is impaired at the cuboidal stages resulting in reduced fertility. Also, adult mutant females develop ovarian cysts and ovarian tubular hyperplasia. Therefore, a block in Kit receptor-mediated PI 3-kinase signaling may be compensated for in hematopoiesis, melanogenesis and primordial germ cell development, but is critical in spermatogenesis and oogenesis.
Figures
Fig. 1. Targeted mutation of Tyr719 in the 129/Sv Kit locus does not affect pigmentation in _Kit_Y719F/_Kit_Y719F mice. (A) Schematic representation of targeting strategy. B, _Bam_HI; E, _Eco_RI; Xh, _Xho_I. LoxP sites are indicated by triangles. (B) Southern blot analysis of tail tips using _Bam_HI and _Eco_RI digestion [see (A) for detail]. (C) Determination of cell surface expression of Kit in _Kit_Y719F/_Kit_Y719F, _Kit_Y719F/neo/_Kit_Y719F/neo and control BMMCs using FACS. The pigmentation phenotype of +/+, _Kit_Y719F/neo/_Kit_Y719F/neo and _Kit_Y719F/_Kit_Y719F mice is shown in the bottom panel.
Fig. 1. Targeted mutation of Tyr719 in the 129/Sv Kit locus does not affect pigmentation in _Kit_Y719F/_Kit_Y719F mice. (A) Schematic representation of targeting strategy. B, _Bam_HI; E, _Eco_RI; Xh, _Xho_I. LoxP sites are indicated by triangles. (B) Southern blot analysis of tail tips using _Bam_HI and _Eco_RI digestion [see (A) for detail]. (C) Determination of cell surface expression of Kit in _Kit_Y719F/_Kit_Y719F, _Kit_Y719F/neo/_Kit_Y719F/neo and control BMMCs using FACS. The pigmentation phenotype of +/+, _Kit_Y719F/neo/_Kit_Y719F/neo and _Kit_Y719F/_Kit_Y719F mice is shown in the bottom panel.
Fig. 1. Targeted mutation of Tyr719 in the 129/Sv Kit locus does not affect pigmentation in _Kit_Y719F/_Kit_Y719F mice. (A) Schematic representation of targeting strategy. B, _Bam_HI; E, _Eco_RI; Xh, _Xho_I. LoxP sites are indicated by triangles. (B) Southern blot analysis of tail tips using _Bam_HI and _Eco_RI digestion [see (A) for detail]. (C) Determination of cell surface expression of Kit in _Kit_Y719F/_Kit_Y719F, _Kit_Y719F/neo/_Kit_Y719F/neo and control BMMCs using FACS. The pigmentation phenotype of +/+, _Kit_Y719F/neo/_Kit_Y719F/neo and _Kit_Y719F/_Kit_Y719F mice is shown in the bottom panel.
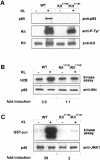
**Fig. 2.**KL/Mgf-stimulated activation of Akt and JNK in _Kit_Y719F/_Kit_Y719F BMMCs. (A) Phosphorylation of Kit receptors in _Kit_Y719F/_Kit_Y719F BMMCs in response to KL/Mgf and association with the p85 subunit of PI 3–kinase. Cells were starved for 12 h in serum-free medium and then stimulated with KL/Mgf for 5 min. Middle panel: Kit proteins were immunoprecipitated, fractionated by SDS–PAGE and blotted with anti-phosphotyrosine antibody. Upper panel: membranes were stripped and reblotted with the anti-p85 antibody. Lower panel: Kit protein levels are shown. (B) _Kit_Y719F/_Kit_Y719F and wild-type BMMCs were starved in a serum-free medium for 12 h, followed by stimulation with KL/Mgf for 5 min. Cells were lysed and an Akt in vitro kinase assay was performed using histone H2B as a substrate. Lower panel: Akt protein (p60) levels are shown. (C) _Kit_Y719F/_Kit_Y719F and wild-type BMMCs were starved in a serum-free medium for 12 h and subsequently stimulated with KL/Mgf for 15 min and a JNK in vitro kinase assay was performed using GST–Jun fusion protein as a substrate. Lower panel: JNK1 protein (p46) levels are shown. The relative phosphorylation was quantitated using a phosphoimage analyzer (Fuji Mac Bas).
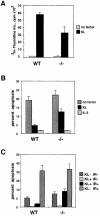
Fig. 3. Kit-mediated proliferation and suppression of apoptosis are affected in _Kit_Y719F/_Kit_Y719F BMMCs. (A) Proliferation. _Kit_Y719F/_Kit_Y719F and wild-type BMMCs were pretreated in a serum-free medium containing IL–3 (20 ng/ml) for 12 h, then starved for 1 h without factors; KL/Mgf (200 ng/ml) or IL–3 (20 ng/ml) was added and after 24 h [3H]thymidine incorporation was determined as described. (B) Deprivation-induced apoptosis. _Kit_Y719F/_Kit_Y719F and wild-type BMMCs were pretreated as in (A), deprived of growth factors for 50 h and analyzed as described. (C) Irradiation-induced apoptosis. _Kit_Y719F/_Kit_Y719F and wild-type BMMCs were pretreated as in (A), subjected to γ–irradiation (25 Gy) or left untreated in the presence or absence of KL/Mgf (200 ng/ml). After 24 h cells were harvested and analyzed for apoptosis.
Fig. 4. Histological analysis of postnatal testis in _Kit_Y719F/_Kit_Y719F mice. Paraffin sections obtained from mutant (b, d, f, h, j, l, n and p) and wt (a, c, e, g, i, k, m and o) testis were stained with H&E. P7 (a and b) and (c and d); closed arrows identify spermatogonia at the basal membrane of tubules (d). Six mutant and four control animals were analyzed at P6/P7. At P10 no meiotic cells are found in mutant testis (e–h). The closed arrow in (g) identifies a primary spermatocyte. In mutant testis, spermatogonia at different stages of development are present. An open arrow identifies dividing spermatogonia in (f) and type A spermatogonia in (h). Mutant testis shows an increased frequency of apoptotic cells (closed arrows in f and h). At P10/P12 four mutant and three control animals were analyzed. At P21 _Kit_Y719F/_Kit_Y719F seminiferous tubules are empty and only a few germ cells are at the base of tubules (i–l). An increase in the interstitial space in mutant testis is detectable (closed arrows in j). At P21 two mutants and one control were analyzed. Adult _Kit_Y719F/_Kit_Y719F testis (16w) are significantly smaller than controls (m and n). Clusters of cells in the center of seminiferous tubules (closed arrows in p) are prominent and the interstitial space is enlarged by hyperplastic Leydig cells (open arrow in p). Five mutant adults and three controls were analyzed. Magnifications are 5× (m and n), 20× (i, j, o and p), 40× (a, b, e, f, k and l) and 100× (c, d, g and h).
Fig. 5. Identification of mitotically active and apoptotic germ cells in _Kit_Y719F/_Kit_Y719F and wt testis. TUNEL analysis was performed on paraffin sections of P10 wt (a) and mutant (b) testis. Ki–67 staining specific for proliferating cells was performed on paraffin sections of wt (c and e) and mutant (d and f) testis at P12 (c and d) and 16 weeks (e and f). Closed arrows in (e) and (f) show nuclei of Leydig cells in wt (e) and _Kit_Y719F/_Kit_Y719F (f) testis. Expression of Kit mRNA in 16 week wt and _Kit_Y719F/_Kit_Y719F testis examined by in situ hybridization (g–j). Dark field images of wt and _Kit_Y719F/_Kit_Y719F testis (g and i) show uniform Kit expression in the interstitial space (white grains). Corresponding bright field images are shown (h and j). Magnifications are 20× (a, b, c and d), 100× (e and f) and 10× (g, h, i and j).
Fig. 6. Histological analysis of postnatal ovaries in _Kit_Y719F/_Kit_Y719F mice. Kit mRNA expression in wt and mutant ovaries was determined by in situ hybridization. The mutation does not affect Kit mRNA expression in oocytes of P17 ovaries and comparable levels of Kit are observed in oocytes of _Kit_Y719F/_Kit_Y719F (a) and wt (b) ovaries. In the dark field images (a and b) closed arrows show similar hybridization in antral follicles. Paraffin-embedded sections obtained from _Kit_Y719F/_Kit_Y719F (d, f, h, j and l) and wt (c, e, g, i and k) ovaries were stained with H&E. Follicle development in _Kit_Y719F/_Kit_Y719F mutant females is delayed at the cuboidal stages and the number of mature follicles is greatly reduced. At P7 there are fewer growing follicles in mutant ovaries (d) compared with wt (c). At P17 (e, f, g and h) an increase in the number of primordial/primary type 2 (closed arrow) and type 3a (open arrow) follicles is seen in the cortex of the ovary of mutant mice compared with wt. Growing follicles in _Kit_Y719F/_Kit_Y719F ovaries are typically surrounded by one or two layers of granulosa cells (f and h). A few follicles escape the defect and mature to antral stages (open arrow in j), as seen in the adult ovary (16 weeks) (i, j, k and l). Oocytes in the cortex of mutant ovaries are degenerated, acentric and follicle development is arrested at the type 3b stage (l). Follicles in the cortex of wt ovary are shown in (k). Solid arrows identify primordial and primary follicles in (j) and (l). The sections shown are representative of eight mutant and eight control animals at the juvenile stages and of six adult mutant and three adult control animals. Magnifications are 5× (i and j), 10× (e and f), 20× (a, b, c and d) and 40× (g, h, k and l).
Fig. 7. Identification of ovarian cysts and tubular hyperplasia in adult _Kit_Y719F/_Kit_Y719F ovaries. H&E staining of paraffin sections of 18–week-old wt (a) and mutant ovaries at 16 (b), 18 (c–e) and 32 weeks (f and g) are shown. Only portions of the ovarian cortex at 16–18 weeks contained follicles and follicle-like structures (b and c). Large regions of the ovary are occupied by luteinized interstitial cells (asterisk in b). The oocyte-depleted ovaries have a high incidence in the development of ovarian cysts (closed arrows in c) and in one case an ovarian tumor has been observed (d); detail of the tumor (rectangle) is shown at high magnification in (e). At 32 weeks the ovary represents a mixture of follicle-like structures and somatic cells surrounding disorganized groups of interstitial and luteal-like cells (f). Invagination of the germinal epithelium (g, closed arrow) and tubular hyperplasia can be observed (f and g). Open arrows in (g) show invading epithelial cells. Magnifications are 5× (a), 10× (c, d and f), 20× (b) and 40× (e and g).
Similar articles
- A role for kit receptor signaling in Leydig cell steroidogenesis.
Rothschild G, Sottas CM, Kissel H, Agosti V, Manova K, Hardy MP, Besmer P. Rothschild G, et al. Biol Reprod. 2003 Sep;69(3):925-32. doi: 10.1095/biolreprod.102.014548. Epub 2003 May 28. Biol Reprod. 2003. PMID: 12773427 - Critical role for Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development.
Agosti V, Corbacioglu S, Ehlers I, Waskow C, Sommer G, Berrozpe G, Kissel H, Tucker CM, Manova K, Moore MA, Rodewald HR, Besmer P. Agosti V, et al. J Exp Med. 2004 Mar 15;199(6):867-78. doi: 10.1084/jem.20031983. J Exp Med. 2004. PMID: 15024050 Free PMC article. - Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3'-kinase is essential for male fertility.
Blume-Jensen P, Jiang G, Hyman R, Lee KF, O'Gorman S, Hunter T. Blume-Jensen P, et al. Nat Genet. 2000 Feb;24(2):157-62. doi: 10.1038/72814. Nat Genet. 2000. PMID: 10655061 - The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis.
Besmer P, Manova K, Duttlinger R, Huang EJ, Packer A, Gyssler C, Bachvarova RF. Besmer P, et al. Dev Suppl. 1993:125-37. Dev Suppl. 1993. PMID: 7519481 Review. - Transcriptional control of KIT gene expression during germ cell development.
Rossi P. Rossi P. Int J Dev Biol. 2013;57(2-4):179-84. doi: 10.1387/ijdb.130014pr. Int J Dev Biol. 2013. PMID: 23784828 Review.
Cited by
- Modeling mammalian spermatogonial differentiation and meiotic initiation in vitro.
Kirsanov O, Johnson T, Malachowski T, Niedenberger BA, Gilbert EA, Bhowmick D, Ozdinler PH, Gray DA, Fisher-Wellman K, Hermann BP, Geyer CB. Kirsanov O, et al. Development. 2022 Nov 15;149(22):dev200713. doi: 10.1242/dev.200713. Epub 2022 Nov 16. Development. 2022. PMID: 36250451 Free PMC article. - Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse.
Busada JT, Chappell VA, Niedenberger BA, Kaye EP, Keiper BD, Hogarth CA, Geyer CB. Busada JT, et al. Dev Biol. 2015 Jan 1;397(1):140-9. doi: 10.1016/j.ydbio.2014.10.020. Epub 2014 Nov 4. Dev Biol. 2015. PMID: 25446031 Free PMC article. - Effects of glucagon-like peptide-1 receptor agonists on spermatogenesis-related gene expression in mouse testis and testis-derived cell lines.
Iida M, Asano A. Iida M, et al. J Vet Med Sci. 2024 May 25;86(5):555-562. doi: 10.1292/jvms.24-0042. Epub 2024 Apr 1. J Vet Med Sci. 2024. PMID: 38556323 Free PMC article. - Protein Tyrosine Phosphatase PRL2 Mediates Notch and Kit Signals in Early T Cell Progenitors.
Kobayashi M, Nabinger SC, Bai Y, Yoshimoto M, Gao R, Chen S, Yao C, Dong Y, Zhang L, Rodriguez S, Yashiro-Ohtani Y, Pear WS, Carlesso N, Yoder MC, Kapur R, Kaplan MH, Daniel Lacorazza H, Zhang ZY, Liu Y. Kobayashi M, et al. Stem Cells. 2017 Apr;35(4):1053-1064. doi: 10.1002/stem.2559. Epub 2017 Jan 19. Stem Cells. 2017. PMID: 28009085 Free PMC article. - Disruption of PDGFRalpha-initiated PI3K activation and migration of somite derivatives leads to spina bifida.
Pickett EA, Olsen GS, Tallquist MD. Pickett EA, et al. Development. 2008 Feb;135(3):589-98. doi: 10.1242/dev.013763. Development. 2008. PMID: 18192285 Free PMC article.
References
- Alai M., Mui, A.L., Cutler, R.L., Bustelo, X.R., Barbacid, M. and Krystal, G. (1992) Steel factor stimulates the tyrosine phosphorylation of the proto-oncogene product, p95vav, in human hemopoietic cells. J. Biol. Chem., 267, 18021–18025. - PubMed
- Bachvarova R.F., Manova,K. and Besmer,P. (1993) Role in gametogenesis of c-kit encoded at the W locus of mice. In Bernfield,M. (ed.), Molecular Basis of Morphogenesis. Wiley-Liss, New York, NY.
- Bennett D. (1956) Developmental analysis of a mutation with pleiotropic effects in the mouse. J. Morphol., 98, 199–229.
- Besmer P. (1997) Kit-ligand-stem cell factor. In Garland,J.M., Quesenberry,P.J. and Hilton,D.J. (eds), Colony-Stimulating Factors: Molecular and Cellular Biology. Marcel Dekker, New York, NY.
- Besmer P., Manova,K., Duttlinger,R., Huang,E.J., Packer,A., Gyssler,C. and Bachvarova,R.F. (1993) The kit-ligand (steel factor) and its receptor c-kit/W: pleiotropic roles in gametogenesis and melanogenesis. Dev. Suppl., 125–137. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous