Cyclin F regulates the nuclear localization of cyclin B1 through a cyclin-cyclin interaction - PubMed (original) (raw)
Cyclin F regulates the nuclear localization of cyclin B1 through a cyclin-cyclin interaction
M Kong et al. EMBO J. 2000.
Abstract
The key regulator of G(2)-M transition of the cell cycle is M-phase promoting factor (MPF), a complex composed of cdc2 and a B-type cyclin. Cyclin B1 nuclear localization involves phosphorylation within a region called the cytoplasmic retention signal, which also contains a nuclear export signal. The mechanism of MPF nuclear localization remains unclear since it contains no functional nuclear localization signal (NLS). We exploited the yeast two-hybrid screen to find protein(s) potentially mediating localization of cyclin B1 and identified a novel interaction between cyclin B1 and cyclin F. We found that cdc2, cyclin B1 and cyclin F form a complex that exhibits histone H1 kinase activity. Cyclin B1 and cyclin F also colocalize through immunofluorescence studies. Additionally, deletion analysis revealed that each putative NLS of cyclin F is functional. Taken together, the data suggest that the NLS regions of cyclin F regulate cyclin B1 localization to the nucleus. The interaction between cyclin B1 and cyclin F represents the first example of direct cyclin-cyclin binding, and elucidates a novel mechanism that regulates MPF localization and function.
Figures
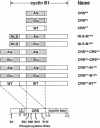
Fig. 1. Schematic representation of Xenopus cyclin B1-derived fusion proteins. The single and double CRS derivatives of cyclin B1 (aa 78–127) contained either all Ala or Glu mutations at residues 94, 96, 101 and 113. The NLS–B1 derivatives contained a nuclear localization signal appended to the N–terminus of cyclin B1. The CRS-B1 derivatives contained an additional CRS region appended to full-length cyclin B1.
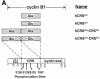
Fig. 2. Human cyclin B1 and cyclin F constructs. (A) The CRS region of human cyclin B1 was synthesized with the Ser residues at 126, 128, 133 and 147 all mutated to either Ala or Glu. (B) Myristylated human cyclin F (top), ΔNLS1/2-cyclin F (middle) and cyclin F (bottom) are shown. The 188 aa region of mouse cyclin F that interacted with the CRS of cyclin B1 corresponding to human cyclin F is indicated at the bottom.
Fig. 2. Human cyclin B1 and cyclin F constructs. (A) The CRS region of human cyclin B1 was synthesized with the Ser residues at 126, 128, 133 and 147 all mutated to either Ala or Glu. (B) Myristylated human cyclin F (top), ΔNLS1/2-cyclin F (middle) and cyclin F (bottom) are shown. The 188 aa region of mouse cyclin F that interacted with the CRS of cyclin B1 corresponding to human cyclin F is indicated at the bottom.
Fig. 3. Expression of cyclin F RNA during Xenopus development. Total RNA isolated from stage VI oocytes or staged embryos was isolated and analyzed by RT–PCR. RNA (200 ng) was assayed for either the presence of cyclin F (upper panel) or, as an internal control, c–src RNA (lower panel).
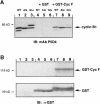
Fig. 4. In vitro binding of GST–cyclin F and Xenopus cyclin B1 fusion proteins. (A) Xenopus cyclin B1 derivatives were translated in reticulocyte lysates (lanes 1, 2 and 3) and then incubated with GST protein alone (lanes 4, 5 and 6) or with bacterially expressed GST–cyclin F (lanes 7, 8 and 9) bound to glutathione–agarose beads. The samples were analyzed by 7.5% SDS–PAGE and immunoblotted with mAb P5D4 directed against the epitope-tag VSV-G on each cyclin B1 derivative. (B) The membrane was re-probed with α–GST antibody, indicating the presence of GST protein (lanes 4, 5 and 6) and GST–cyclin F (lanes 7, 8 and 9). Molecular mass markers in kilodaltons are indicated.
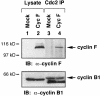
Fig. 5. cdc2, cyclin B1 and cyclin F form a complex. Cyclin F was transfected into 293T cells and then immunoprecipitated with α–cdc2 antibody. The samples were analyzed by 10% SDS–PAGE followed by immunoblotting with α–cyclin F antibody (upper panel) and α–cyclin B1 (lower panel). The lower band in the immunoprecipitated samples in lanes 3 and 4 of the lower panel represents the IgG band from the α–cdc2 antibody. Molecular mass markers are indicated.
Fig. 6. Interaction of endogenous cyclin F with endogenous cyclin B1. (A) HeLa cells were synchronized by double-thymidine block to G2–M phase, and the lysate was immunoprecipitated with α–cyclin B1 antibody (lane 2) or α–cyclin B1 plus α–cyclin B1 blocking peptide (lane 3). Samples were analyzed by 10% SDS–PAGE followed by immunoblotting with α–cyclin F antibody (upper panel) and α–cyclin B1 antibody (lower panel). The lysate served as a positive control (lane 1). (B) Synchronized HeLa cells in G2–M were lysed and immunoprecipitated with α–cdc2 antibody (lane 2). Analysis by 10% SDS–PAGE followed by immunoblotting revealed the presence of cyclin F (upper panel), cyclin B1 (middle panel) and cdc2 (lower panel). The lysate served as a positive control (lane 1). The lower band in the cyclin B1 immunoblot of the α–cdc2 immunoprecipitate (lane 2) is due to the IgG band of the α–cdc2 antibody. (C) HeLa cells were lysed and immunoprecipitated with α–cyclin F (lane 1), α–cyclin F plus α–cyclin F blocking peptide (lane 2), or α–cdc2 antibody (lane 3). Samples were then assayed for histone H1 kinase activity. Molecular mass markers in all panels are indicated.
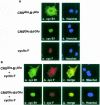
Fig. 7. (A) Individual localization of CRS-cyclin B1 and cyclin F in COS–1 cells synchronized in G2–M phase. Panels a and c are CRSAla-B1Ala and CRSGlu-B1Glu, respectively, fixed and stained with mAb P5D4 (green). Panel e shows cyclin F fixed and stained with α–cyclin F antibody (red). Hoechst dye 33342 reveals the nucleus in panels b, d and f (blue). (B) Colocalization of CRS-cyclin B1 and cyclin F. The CRSAla-B1Ala and cyclin F cotransfection (top row) shows that the cyclin B1 Ala mutant (green) and cyclin F (red) colocalize (yellow) in the cytoplasm. The CRSGlu-B1Glu and cyclin F cotransfection (bottom row) indicates that the cyclin B1 Glu mutant (green) and cyclin F (red) also colocalize (yellow), but in the nucleus.
Fig. 8. (A) Individual localization of NLS–B1 and myr-cyclin F in COS–1 cells synchronized in G2–M phase. Panels a and c are NLS–B1 derivatives stained with mAb P5D4 (green). Panel e is myristylated cyclin F stained with α–cyclin F antibody (red). Hoechst dye shows the nucleus in panels b, d and f (blue). (B) Colocalization of NLS–B1 and myr-cyclin F. The NLS–B1Ala and myr-cyclin F cotransfection (top row) shows that the NLS–B1Ala mutant (green) and myr-cyclin F (red) colocalize (yellow). The NLS–B1Glu and myr-cyclin F cotransfection indicates that the NLS–B1Glu mutant (green) and myr-cyclin F (red) colocalize (yellow).
Fig. 9. ΔNLS1/2-cyclin F localizes to the cytoplasm and retains NLS–B1Glu in the cytoplasm. (A) ΔNLS1/2-cyclin F transfected cells were fixed and stained with an antibody directed against the epitope-tag of cyclin F (panel a, red). The nucleus is seen with Hoechst dye (panel b, blue) and indicates that ΔNLS1/2-cyclin F is completely excluded from the nucleus. (B) NLS–B1Glu (panel c, green) is cotransfected with ΔNLS1/2-cyclin F (panel d, red) and they colocalize (panel e, yellow).
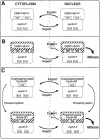
Fig. 10. Model of cyclin B1–cyclin F colocalization. (A) CRSAla-B1Ala and cyclin F colocalize in the cytoplasm, undergo nuclear transport, and then re-localize to the cytoplasm due to the functional NES of CRSAla-B1Ala. (B) CRSGlu-B1Glu and cyclin F interact in the cytoplasm and then colocalize to the nucleus. The complex is then retained in the nucleus due to the inactive NES of CRSGlu-B1Glu and properly localized to trigger the onset of mitosis. (C) Upper panel: unphosphorylated cyclin B1 and cyclin F interact in the cytoplasm and enter the nucleus. Re-localization to the cytoplasm may then occur via the active NES of cyclin B1. Lower panel: phosphorylated cyclin B1 and cyclin F colocalize from the cytoplasm to the nucleus. MPF is active and ready to initiate mitosis. In each panel, cdc2 is complexed to cyclin B1, although it is not represented.
Similar articles
- Nuclear localization of cyclin B1 mediates its biological activity and is regulated by phosphorylation.
Li J, Meyer AN, Donoghue DJ. Li J, et al. Proc Natl Acad Sci U S A. 1997 Jan 21;94(2):502-7. doi: 10.1073/pnas.94.2.502. Proc Natl Acad Sci U S A. 1997. PMID: 9012813 Free PMC article. - MPF localization is controlled by nuclear export.
Hagting A, Karlsson C, Clute P, Jackman M, Pines J. Hagting A, et al. EMBO J. 1998 Jul 15;17(14):4127-38. doi: 10.1093/emboj/17.14.4127. EMBO J. 1998. PMID: 9670027 Free PMC article. - Polo-like kinase 1 phosphorylates cyclin B1 and targets it to the nucleus during prophase.
Toyoshima-Morimoto F, Taniguchi E, Shinya N, Iwamatsu A, Nishida E. Toyoshima-Morimoto F, et al. Nature. 2001 Mar 8;410(6825):215-20. doi: 10.1038/35065617. Nature. 2001. PMID: 11242082 - Cyclin B1 and CDK1: nuclear localization and upstream regulators.
Porter LA, Donoghue DJ. Porter LA, et al. Prog Cell Cycle Res. 2003;5:335-47. Prog Cell Cycle Res. 2003. PMID: 14593728 Review. - 14-3-3 proteins: regulation of subcellular localization by molecular interference.
Muslin AJ, Xing H. Muslin AJ, et al. Cell Signal. 2000 Dec;12(11-12):703-9. doi: 10.1016/s0898-6568(00)00131-5. Cell Signal. 2000. PMID: 11152955 Review.
Cited by
- Polyplex exposure inhibits cell cycle, increases inflammatory response, and can cause protein expression without cell division.
Matz RL, Erickson B, Vaidyanathan S, Kukowska-Latallo JF, Baker JR Jr, Orr BG, Banaszak Holl MM. Matz RL, et al. Mol Pharm. 2013 Apr 1;10(4):1306-17. doi: 10.1021/mp300470d. Epub 2013 Mar 21. Mol Pharm. 2013. PMID: 23458572 Free PMC article. - Three distinct domains contribute to nuclear transport of murine Foxp3.
Hancock WW, Ozkaynak E. Hancock WW, et al. PLoS One. 2009 Nov 18;4(11):e7890. doi: 10.1371/journal.pone.0007890. PLoS One. 2009. PMID: 19924293 Free PMC article. - A Novel Signature of CCNF-Associated E3 Ligases Collaborate and Counter Each Other in Breast Cancer.
Chang SC, Hung CS, Zhang BX, Hsieh TH, Hsu W, Ding JL. Chang SC, et al. Cancers (Basel). 2021 Jun 8;13(12):2873. doi: 10.3390/cancers13122873. Cancers (Basel). 2021. PMID: 34201347 Free PMC article. - Mitotic kinase anchoring proteins: the navigators of cell division.
Fulcher LJ, Sapkota GP. Fulcher LJ, et al. Cell Cycle. 2020 Mar;19(5):505-524. doi: 10.1080/15384101.2020.1728014. Epub 2020 Feb 12. Cell Cycle. 2020. PMID: 32048898 Free PMC article. Review. - Distinct gene expression profiles in different B-cell compartments in human peripheral lymphoid organs.
Shen Y, Iqbal J, Xiao L, Lynch RC, Rosenwald A, Staudt LM, Sherman S, Dybkaer K, Zhou G, Eudy JD, Delabie J, McKeithan TW, Chan WC. Shen Y, et al. BMC Immunol. 2004 Sep 15;5:20. doi: 10.1186/1471-2172-5-20. BMC Immunol. 2004. PMID: 15369600 Free PMC article.
References
- Bai C., Sen, P., Hofmann, K., Ma, L., Goebl, M., Harper, J.W. and Elledge, S.J. (1996) SKP1 connects cell cycle regulators to the ubiquitin proteolysis machinery through a novel motif, the F–box. Cell, 86, 263–274. - PubMed
- Booher R.N., Alfa, C.E., Hyams, J.S. and Beach, D.H. (1989) The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell, 58, 485–497. - PubMed
- Boulikas T. (1996) Nuclear import of protein kinases and cyclins. J. Cell Biol., 60, 61–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous