The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus - PubMed (original) (raw)
The tyrosine kinase and mitogen-activated protein kinase pathways mediate multiple effects of estrogen in hippocampus
R Bi et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Estrogen replacement therapy in women is associated with improvement of cognitive deficits and reduced incidence of Alzheimer's disease. The present study indicates that estrogen is neuroprotective against N-methyl-d-aspartate (NMDA)- and kainate-mediated neurotoxicity, an effect mediated by tyrosine kinase/mitogen-activated protein kinase (MAPK) pathways. Estrogen also stimulates tyrosine phosphorylation of NMDA receptors via an src tyrosine kinase/MAPK pathway. Finally, estrogen-mediated enhancement of long-term potentiation in hippocampal slices is mediated by activation of an src tyrosine kinase pathway. Thus, estrogen, by activating an src tyrosine kinase and the extracellular signal-related protein kinase/MAPK signaling pathway, both enhances NMDA receptor function and long-term potentiation and retains neuroprotective properties against excitotoxicity. These findings warrant further evaluation of the usefulness of estrogenic compounds for the treatment of Alzheimer's disease and other neurodegenerative diseases.
Figures
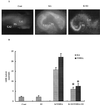
Figure 1
Effects of E2 on KA- and NMDA-elicited neurotoxicity. Hippocampal slices were prepared as described in Materials and Methods and were maintained in cultures for 10–14 days. They were treated with NMDA or KA (50 μM) for 3 h and returned to normal medium for 24 h. When present, E2 was added at 1 nM 24 h before NMDA or KA and was present for the duration of the experiment. (A) PI uptake in control, 24 h after a 3-h treatment with KA (50 μM) in the absence or presence of E2. DG, dentate gyrus. (B) LDH activity in medium measured in control (Cont), 48 h after treatment with E2 (E2), 24 h after KA or NMDA treatment in the absence (K/NMDA) or presence of E2 (K+E2/NMDA+E2). Results are means ± SEM of six to eight experiments. *, P < 0.001 compared with KA or NMDA (Student's t test).
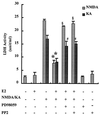
Figure 2
Effects of tyrosine kinase inhibitors on E2-mediated neuroprotection against KA and NMDA neurotoxicity. Hippocampal slices were treated as described for Fig. 1. PP2 (10 μM) or PD98059 (50 μM) was added at the same time and for the same duration as E2. Toxicity was assessed by measuring LDH release in the medium 24 h after KA or NMDA treatment. Results are means ± SEM of six to eight experiments. *, P < 0.001 compared with KA or NMDA alone; §, not significantly different from NMDA alone; #, not significantly different from KA alone (Student's t test).
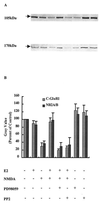
Figure 3
Effects of tyrosine kinase inhibitors on E2-mediated protection of the C-terminal domain of GluR1 or NR2 subunits against NMDA-elicited truncation. Hippocampal slices were treated with NMDA (50 μM) for 3 h. E2 and PP2 or PD98059 were added as described for Fig. 2. Immunoblots were performed as described in Materials and Methods and were labeled with antibodies against the C-terminal domains of GluR1 (105 kDa) or NR2 (170 kDa) subunits. (A) Representative blot, with the order of treatment as indicated in B. (B) Quantification of blots similar to the ones shown in A. Results are expressed as the percentage of values found in control conditions and are means ± SEM of six to eight experiments.
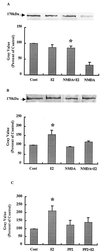
Figure 4
Effects of E2 on NR2 subunits of NMDA receptors. Hippocampal slices were treated as described for Fig. 3. At the end of the experiments, membrane fractions were prepared, and proteins were immunoprecipitated with anti-NR2A/B antibodies. Immunoblots were then stained with antibodies against anti-NR2A/B (A) or anti-phosphotyrosine (B). Alternatively, slices were incubated for 30 min with E2 in the presence or absence of PP2 (10 μM). Immunoblots of samples from membrane fractions were stained with anti-phosphotyrosine antibodies and quantified as described (C). (A Upper) Representative Western blot. (A Lower) Quantitative analysis of blots similar to the blot shown in A Upper. Results are expressed as percentage of values found in control conditions (Cont) and are means ± SEM of four experiments. *, P < 0.001 as compared with control (Student's t test). (B Upper) Representative Western blot. (B Lower) Quantitative analysis of blots similar to the blot shown in B Upper. Results are expressed as percentage of values found in control conditions (Cont) and are means ± SEM of four experiments. *, P < 0.001 compared with control (Student's t test). (C) Quantitative analysis of immunoblots of membrane fractions stained with anti-phosphotyrosine antibodies. Results are expressed as percentage of values found in control conditions (Cont) and are means ± SEM of four experiments. *, P < 0.001 compared with control (Student's t test).
Figure 5
Effects of an src inhibitor on E2-mediated enhancement of EPSP amplitude and degree of LTP in acute hippocampal slices. Acute hippocampal slices were prepared as described in Materials and Methods. A stimulating electrode was located in CA3, and a recording electrode was located in stratum radiatum of CA1. Extracellular EPSPs were evoked by stimulation every 30 s, and the amplitude of the EPSP was measured. After a stable baseline was recorded, PP2 (10 μM) was added at the indicated time. Likewise, E2 (1 nM) was added at the indicated time. After resetting the stimulation intensity to obtain an EPSP of the same amplitude as before treatment with PP2 or E2, high frequency stimulation was delivered, and low frequency stimulation was resumed. Results are expressed as percentage of predrug values and are means ± SEM of 6–10 experiments. HFS, high-frequency stimulation.
Similar articles
- Augmentation by zinc of NMDA receptor-mediated synaptic responses in CA1 of rat hippocampal slices: mediation by Src family tyrosine kinases.
Kim TY, Hwang JJ, Yun SH, Jung MW, Koh JY. Kim TY, et al. Synapse. 2002 Nov;46(2):49-56. doi: 10.1002/syn.10118. Synapse. 2002. PMID: 12211081 - Neuronal protein kinase signaling cascades and excitotoxic cell death.
Skaper SD, Facci L, Strijbos PJ. Skaper SD, et al. Ann N Y Acad Sci. 2001 Jun;939:11-22. doi: 10.1111/j.1749-6632.2001.tb03606.x. Ann N Y Acad Sci. 2001. PMID: 11462762 Review. - The role of non-receptor protein tyrosine kinases in the excitotoxicity induced by the overactivation of NMDA receptors.
Sun Y, Chen Y, Zhan L, Zhang L, Hu J, Gao Z. Sun Y, et al. Rev Neurosci. 2016 Apr 1;27(3):283-9. doi: 10.1515/revneuro-2015-0037. Rev Neurosci. 2016. PMID: 26540220 Review.
Cited by
- Estrogen Signaling as a Therapeutic Target in Neurodevelopmental Disorders.
Crider A, Pillai A. Crider A, et al. J Pharmacol Exp Ther. 2017 Jan;360(1):48-58. doi: 10.1124/jpet.116.237412. Epub 2016 Oct 27. J Pharmacol Exp Ther. 2017. PMID: 27789681 Free PMC article. Review. - Hippocampal synthesis of sex steroids and corticosteroids: essential for modulation of synaptic plasticity.
Hojo Y, Higo S, Kawato S, Hatanaka Y, Ooishi Y, Murakami G, Ishii H, Komatsuzaki Y, Ogiue-Ikeda M, Mukai H, Kimoto T. Hojo Y, et al. Front Endocrinol (Lausanne). 2011 Oct 10;2:43. doi: 10.3389/fendo.2011.00043. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22701110 Free PMC article. - Involvement of BDNF receptor TrkB in spatial memory formation.
Mizuno M, Yamada K, He J, Nakajima A, Nabeshima T. Mizuno M, et al. Learn Mem. 2003 Mar-Apr;10(2):108-15. doi: 10.1101/lm.56003. Learn Mem. 2003. PMID: 12663749 Free PMC article. - Estrogen alters spinal NMDA receptor activity via a PKA signaling pathway in a visceral pain model in the rat.
Tang B, Ji Y, Traub RJ. Tang B, et al. Pain. 2008 Jul 31;137(3):540-549. doi: 10.1016/j.pain.2007.10.017. Epub 2008 Feb 20. Pain. 2008. PMID: 18068901 Free PMC article. - Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-β.
Aguirre C, Jayaraman A, Pike C, Baudry M. Aguirre C, et al. J Neurochem. 2010 Dec;115(5):1277-87. doi: 10.1111/j.1471-4159.2010.07038.x. Epub 2010 Oct 26. J Neurochem. 2010. PMID: 20977477 Free PMC article.
References
- Henderson V W. Neurology. 1997;48, Suppl. 7:S27–S35. - PubMed
- Schneider L S, Farlow M R, Henderson V W, Pogoda J M. Neurology. 1996;46:1580–1584. - PubMed
- Doraiswamy P M, Bieber F, Kaiser L, Krishnan K R, Reuning-Scherer J, Gulanski B. Neurology. 1997;48:1511–1517. - PubMed
- Simpkins J W, Green P S, Gridley K E, Singh M, de Fiebre N C, Rajakumar G. Am J Med. 1997;103:19S–25S. - PubMed
- Teyler T J, Vardaris R M, Lewis D, Rawitch A B. Science. 1980;209:1017–1019. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous