Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses - PubMed (original) (raw)
. 2000 Apr 25;97(9):4760-5.
doi: 10.1073/pnas.97.9.4760.
Y Nagata, S Gnjatic, H Wada, E Stockert, J Karbach, P R Dunbar, S Y Lee, A Jungbluth, D Jäger, M Arand, G Ritter, V Cerundolo, B Dupont, Y T Chen, L J Old, A Knuth
Affiliations
- PMID: 10781081
- PMCID: PMC18306
- DOI: 10.1073/pnas.97.9.4760
Monitoring CD8 T cell responses to NY-ESO-1: correlation of humoral and cellular immune responses
E Jäger et al. Proc Natl Acad Sci U S A. 2000.
Abstract
NY-ESO-1, a member of the cancer-testis family of antigens, is expressed in a subset of a broad range of different human tumor types. Patients with advanced NY-ESO-1-expressing tumors frequently develop humoral immunity to NY-ESO-1, and three HLA A2-restricted peptides were defined previously as targets for cytotoxic CD8(+) T cells in a melanoma patient with NY-ESO-1 antibody. The objectives of the present study were (i) to develop enzyme-linked immunospot (ELISPOT) and tetramer assays to measure CD8(+) T cell responses to NY-ESO-1, (ii) to determine the frequency of CD8(+) T cell responses to NY-ESO-1 in a series of HLA-A2 patients with NY-ESO-1 expressing tumors, (iii) to determine the relation between CD8(+) T cell and humoral immune responses to NY-ESO-1, and (iv) to compare results of NY-ESO-1 ELISPOT assays performed independently in two laboratories with T cells from the same patients. NY-ESO-1 ELISPOT and tetramer assays with excellent sensitivity, specificity, and reproducibility have been developed and found to correlate with cytotoxicity assays. CD8(+) T cell responses to HLA-A2-restricted NY-ESO-1 peptides were detected in 10 of 11 patients with NY-ESO-1 antibody, but not in patients lacking antibody or in patients with NY-ESO-1-negative tumors. The results of ELISPOT assays were concordant in the two laboratories, providing the basis for standardized monitoring of T cell responses in patients receiving NY-ESO-1 vaccines.
Figures
Figure 1
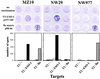
CD8+ T cell reactivity to HLA-A2-restricted peptides in ELISPOT IFN-γ assays. (A) CD8+ T cells from NW731, a patient with NY-ESO-1-positive melanoma and NY-ESO-1 antibody. (B) CD8+ T cells from NW681, a patient with NY-ESO-1-positive melanoma and no NY-ESO-1 antibody. CD8+ T cells were presensitized with NY-ESO-1 p157–165, Melan-A/MART-1 p26–35, MAGE-3 p271–279, or flu matrix p58–66 and tested against T2 cells pulsed with the peptide panel shown at the bottom of the figure (including NY-ESO-1 p157–167). Effector-to-target cell ratios in these assays were 1:1 (solid bars) and 0.2:1 (hatched bars).
Figure 2
Effect of presensitization of CD8+ T cells with HLA-A2-restricted NY-ESO-1 p157–165 and flu matrix p58–66 on ELISPOT assays. Results with CD8+ T cells from three patients with NY-ESO-1 expressing stage IV melanoma and NY-ESO-1 antibody are shown. Fifty thousand CD8+ T cells, with or without presensitization with NY-ESO-1 p157–165 or flu matrix p58–66, were cocultured with 50,000 T2 cells alone or pulsed with NY-ESO-1 p157–165 or flu matrix p58–66. (Upper) Photographs of wells in an ELISPOT assay. (Lower) Histograms with no presensitization (open bars), presensitization with NY-ESO-1 p157–165 (solid bar), or presensitization with flu matrix p58–66 (hatched bar). Each bar represents average of spots in duplicate wells.
Figure 3
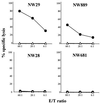
Cytotoxicity tests with CD8+ T cells from four HLA-A2 patients with NY-ESO-1-positive melanomas. Patients NW29 and NW889 had NY-ESO-1 antibody, and patients NW28 and NW681 did not. Cytotoxicity was measured by standard 51Cr release assay, and values represent averages of duplicate wells (▵, T2 cells; ●, T2 cells pulsed with NY-ESO-1 p157–165).
Figure 4
Tetramer analysis of CD8+ T cells from two stage IV melanoma patients, NW29 and NW731, with NY-ESO-1-positive tumors and NY-ESO-1 antibody. Tests with unsensitized PBLs (Upper) and CD8+ T cells presensitized with NY-ESO-1 p157–165 (Lower) for each patient are shown. Samples were double-stained with phycoerythrin-labeled tetramers and anti-Tricolor-CD8 mAb. Tetramers were prepared with NY-ESO-1 p157–165, Melan-A p26–35, and MAGE-3 p271–279 as indicated at the bottom of each grid. Values indicate percentage of tetramer-positive CD8+ T cells.
Similar articles
- The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients.
Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, Qin LL, Fei R, Mei MH, Leng XS, Gnjatic S, Ritter G, Simpson AJ, Old LJ, Chen WF. Shang XY, et al. Clin Cancer Res. 2004 Oct 15;10(20):6946-55. doi: 10.1158/1078-0432.CCR-04-0502. Clin Cancer Res. 2004. PMID: 15501973 - Naturally occurring human lymphocyte antigen-A2 restricted CD8+ T-cell response to the cancer testis antigen NY-ESO-1 in melanoma patients.
Valmori D, Dutoit V, Liénard D, Rimoldi D, Pittet MJ, Champagne P, Ellefsen K, Sahin U, Speiser D, Lejeune F, Cerottini JC, Romero P. Valmori D, et al. Cancer Res. 2000 Aug 15;60(16):4499-506. Cancer Res. 2000. PMID: 10969798 - CD8(+) T cell responses against a dominant cryptic HLA-A2 epitope after NY-ESO-1 peptide immunization of cancer patients.
Gnjatic S, Jäger E, Chen W, Altorki NK, Matsuo M, Lee SY, Chen Q, Nagata Y, Atanackovic D, Chen YT, Ritter G, Cebon J, Knuth A, Old LJ. Gnjatic S, et al. Proc Natl Acad Sci U S A. 2002 Sep 3;99(18):11813-8. doi: 10.1073/pnas.142417699. Epub 2002 Aug 19. Proc Natl Acad Sci U S A. 2002. PMID: 12186971 Free PMC article. - Vaccination for malignant melanoma: recent developments.
Jäger D, Jäger E, Knuth A. Jäger D, et al. Oncology. 2001;60(1):1-7. doi: 10.1159/000055289. Oncology. 2001. PMID: 11150901 Review. - CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: three cases.
Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, Xu Y, Adams S, Bhardwaj N, Busam K, Old LJ, Allison JP, Jungbluth A, Wolchok JD. Yuan J, et al. Cancer Immunol Immunother. 2011 Aug;60(8):1137-46. doi: 10.1007/s00262-011-1011-9. Epub 2011 Apr 5. Cancer Immunol Immunother. 2011. PMID: 21465316 Free PMC article. Review.
Cited by
- Cancer-testis antigen SLLP1 represents a promising target for the immunotherapy of multiple myeloma.
Yousef S, Heise J, Lajmi N, Bartels K, Kröger N, Luetkens T, Atanackovic D. Yousef S, et al. J Transl Med. 2015 Jun 20;13:197. doi: 10.1186/s12967-015-0562-5. J Transl Med. 2015. PMID: 26088750 Free PMC article. - Regulation of cancer germline antigen gene expression: implications for cancer immunotherapy.
Akers SN, Odunsi K, Karpf AR. Akers SN, et al. Future Oncol. 2010 May;6(5):717-32. doi: 10.2217/fon.10.36. Future Oncol. 2010. PMID: 20465387 Free PMC article. Review. - Development and use of multimeric major histocompatibility complex molecules.
Greten TF, Schneck JP. Greten TF, et al. Clin Diagn Lab Immunol. 2002 Mar;9(2):216-20. doi: 10.1128/cdli.9.2.216-220.2002. Clin Diagn Lab Immunol. 2002. PMID: 11874855 Free PMC article. Review. No abstract available. - Identification of tumor antigens as potential target antigens for immunotherapy by serological expression cloning.
Jäger D, Taverna C, Zippelius A, Knuth A. Jäger D, et al. Cancer Immunol Immunother. 2004 Mar;53(3):144-7. doi: 10.1007/s00262-003-0470-z. Epub 2004 Jan 15. Cancer Immunol Immunother. 2004. PMID: 14727084 Free PMC article. Review. - Epigenetic potentiation of NY-ESO-1 vaccine therapy in human ovarian cancer.
Odunsi K, Matsuzaki J, James SR, Mhawech-Fauceglia P, Tsuji T, Miller A, Zhang W, Akers SN, Griffiths EA, Miliotto A, Beck A, Batt CA, Ritter G, Lele S, Gnjatic S, Karpf AR. Odunsi K, et al. Cancer Immunol Res. 2014 Jan;2(1):37-49. doi: 10.1158/2326-6066.CIR-13-0126. Cancer Immunol Res. 2014. PMID: 24535937 Free PMC article. Clinical Trial.
References
- Boon T, Old L J. Curr Opin Immunol. 1997;9:681–683. - PubMed
- van der Bruggen P, Traversari C, Chomez P, Lurquin C, De Plaen E, Van den Eynde B, Knuth A, Boon T. Science. 1991;254:1643–1647. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials