Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor - PubMed (original) (raw)
Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor
H F Farhadi et al. J Neurosci. 2000.
Abstract
Hippocampal neurons release nerve growth factor (NGF) through the constitutive secretory pathway, thus allowing the protein to be continuously available for promoting nerve cell survival. In contrast, hippocampal neurons use the regulated secretory pathway to process brain-derived neurotrophic factor (BDNF), which alters synaptic activity when released acutely from dense-core vesicles. Thus, understanding how neurons sort and deliver neurotrophins may provide clues to their functions in brain. In this study, we monitored the processing and delivery of neurotrophin-3 (NT-3). Pulse-chase studies, immunocytochemistry, and secretagogue-induced release experiments were performed on cultured hippocampal neurons and AtT-20 cells infected with vaccinia viruses encoding the NT-3 precursor (pro-NT-3). Results show that most newly synthesized NT-3 is released through the constitutive secretory pathway as a result of furin-mediated endoproteolytic cleavage of pro-NT-3 in the trans-Golgi network. Pro-NT-3 can also be diverted into the regulated secretory pathway when cells are treated with alpha1-PDX, a selective inhibitor of furin-like enzymes, or when pro-NT-3 expression is increased by transient transfection methods. In cells coinfected with viruses coding for pro-NT-3 and pro-BDNF, NT-3 is sorted into the regulated pathway, stored in secretory granules, and released in response to extracellular cues together with BDNF, apparently as a result of heterodimerization, as suggested by coimmunoprecipitation data. Taken together, these data show that sorting of the NT-3 precursor can occur in both the constitutive and regulated secretory pathways, which is consistent with NT-3 having both survival-promoting and synapse-altering functions.
Figures
Fig. 1.
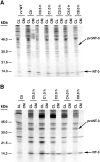
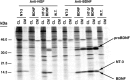
Pulse–chase metabolic labeling of pro-NT-3 in primary cultures of hippocampal neurons (A) and AtT-20 cells (B). Cells were infected with VV encoding the NT-3 precursor for 1 hr and postincubated in fresh medium without virus for 8 hr. Cells were then exposed to medium containing [35S] Cys-Met for 30 min and chased for 0, 0.5, 1, 2, 4, and 8 hr. Identical volumes (750 μl) of cell lysates (CL) and conditioned media (CM) were incubated with antibodies to NGF, which immunoprecipitate NT-3, and electrophoresed on 13–22% SDS gradient gels.
Fig. 2.
Differential processing of neurotrophin precursors in COS-1 cells, which contain only the constitutive secretory pathway. Cells were infected at an MOI of 1 with either wild-type VV (vv:WT) or VV encoding pro-NGF, pro-BDNF, or pro-NT-3. The cells were postincubated in the absence of virus for another 8 hr and metabolically labeled for 3 hr. Identical volumes of cell lysates (CL) and conditioned media (CM) from _vv:WT_-,_vv:NGF_-, and _vv:NT-3_-infected cells were immunoprecipitated with an NGF antibody that recognizes both NGF and NT-3. _vv:BDNF_-infected cells were immunoprecipitated with a BDNF-specific antibody.
Fig. 3.
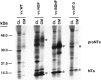
Inhibition of pro-NT-3 processing in AtT-20 cells expressing α1-PDX. AtT-20 cells stably expressing the furin inhibitor α1-PDX were infected for 30 min with VV encoding pro-NT-3. Cells were then incubated in virus-free medium for 8 hr, metabolically labeled for 30 min, and chased for up to 8 hr. Cell lysates (CL) and conditioned media (CM) were immunoprecipitated and analyzed by SDS-PAGE.
Fig. 4.
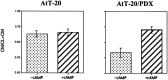
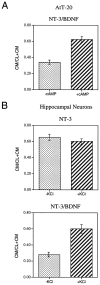
cAMP-induced release of NT-3 from AtT-20 cells expressing α1-PDX. Cells were infected with VV:pro-NT-3 for 1 hr, incubated in virus-free medium for 8 hr, metabolically labeled for 3 hr, chased for 3 hr, and treated for 3 hr with medium with or without 5 m
m
cAMP. CL and CM were immunoprecipitated and the amount of processed, mature NT-3 was analyzed by SDS-PAGE. Analysis was performed on a PhosphorImager, and values represent an average (±SEM) of three independent experiments.
Fig. 5.
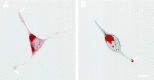
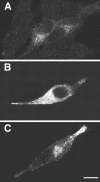
Confocal microscopy of AtT-20 (A) and AtT-20/α1-PDX cells (B) infected with VV encoding pro-NT-3. Cells were infected for 1 hr and postincubated in the absence of virus for another 8 hr. The cultures were fixed and treated with antibodies against NT-3 (Chemicon), followed by CY3-conjugated goat anti-rabbit IgG. Scale bar, 10 μm. Photomicrographs were obtained by overlaying confocal fluorescence images over transmitted light images. Scale bar, 10 μm.
Fig. 6.
Overexpression of NT-3 results in rerouting from the constitutive to the regulated secretory pathway. AtT-20 cells were transfected with a construct encoding either pro-NT-3 (A, B) or pro-BDNF (C), and immunocytochemistry using NT-3 (A, B) and BDNF (C) antibodies was performed as described in Figure 5. In A, cells were lipofectamine-transfected with 0.1 μg of pro-NT-3 DNA (and 1.9 μg vector DNA). In B, cells were transfected with 2 μg pro-NT-3 DNA. In C, cells were transfected with 0.1 μg of pro-BDNF (and 1.9 μg vector DNA). Scale bar, 10 μm.
Fig. 7.
Immunoprecipitation of cell extracts and conditioned medium from cells infected with 1 MOI of wild type (W.T.) VV, VV:pro-NT-3, VV:pro-NGF, VV:pro-BDNF, alone, or coinfected with 0.5 MOI of pro-NT-3 and pro-BDNF. AtT-20 cells were infected for 1 hr with the viruses indicated, postincubated for 8 hr, and metabolically labeled for 3 hr. Cell lysates and conditioned media were immunoprecipitated with either an anti-NGF antibody (left side) or a BDNF antibody (right side).
Fig. 8.
NT-3/BDNF is retained in hippocampal neurons.A, The methodology in Figure 1_A_(involving a 4 hr chase only) was repeated three times with cells infected with either VV:pro-NT-3/VV:WT or VV:pro-NT-3/VV:pro-BDNF. The NGF antibody was used for immunoprecipitations. Results were analyzed on a PhosphorImager and are an average (±SEM) of the ratio of mature NT-3 in cell lysates (CL) over the total amount of NT-3 in CL + conditioned medium (CM).B, A representative SDS gel from the experiments in_A_ showing the NT-3/BDNF heterodimer in the cell lysate and conditioned medium.
Fig. 9.
Secretagogue-induced release of NT-3/BDNF but not NT-3. A, AtT-20 cells coinfected with VV encoding pro-NT-3 and VV encoding pro-BDNF were processed using the methodology described in Figure 4. B, Hippocampal neurons from E18 mice were cultured for 7 d and infected for 1 hr with either (1) VV encoding pro-NT-3 or (2) VV encoding pro-NT-3 and VV encoding pro-BDNF. After 8 hr in medium without virus, the cells were labeled for 30 min with [35S] Cys-Met, incubated in medium without radiolabel for 4 hr, and treated with medium with or without KCl and CaCl2 for 15 min. Cell lysates and conditioned media were immunoprecipitated with the antibody to NGF and electrophoresed on an SDS gel. Results were analyzed on a PhosphorImager and are an average (±SEM) of three independent experiments.
Fig. 10.
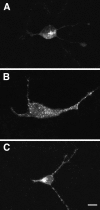
Confocal microscopy of hippocampal neurons infected with pro-NTs. Hippocampal neurons were infected with 1 MOI VV encoding either pro-NT-3 (A) or pro-BDNF (B); in C, the cells were coinfected with 0.5 MOI each of VV:pro-NT-3 and VV:pro-BDNF. Immunocytochemistry was performed with the NT-3 antibody in_A_ and C and the BDNF antibody in_B_. Scale bar, 10 μm.
Fig. 11.
Double-label immunocytochemistry comparing the distribution in infected AtT-20 cells of NT-3 and NT-3/BDNF with that of endogenous TGN38 and ACTH. NT-3 immunoreactivity (A) colocalizes with TGN38 (B) in the perinuclear region as seen in_C_ (NT-3 in red and TGN38 in_green_). NT-3 immunoreactivity in cells coinfected with pro-NT-3 and pro-BDNF (D) colocalizes with ACTH (E) primarily in the tip of the cellular process as seen in F (NT-3 in red and ACTH in_green_). Scale bar, 10 μm.
Similar articles
- Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons.
Mowla SJ, Pareek S, Farhadi HF, Petrecca K, Fawcett JP, Seidah NG, Morris SJ, Sossin WS, Murphy RA. Mowla SJ, et al. J Neurosci. 1999 Mar 15;19(6):2069-80. doi: 10.1523/JNEUROSCI.19-06-02069.1999. J Neurosci. 1999. PMID: 10066260 Free PMC article. - Neurotrophin-4, alone or heterodimerized with brain-derived neurotrophic factor, is sorted to the constitutive secretory pathway.
Hibbert AP, Morris SJ, Seidah NG, Murphy RA. Hibbert AP, et al. J Biol Chem. 2003 Nov 28;278(48):48129-36. doi: 10.1074/jbc.M300961200. Epub 2003 Sep 11. J Biol Chem. 2003. PMID: 12970359 - Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 are sorted to dense-core vesicles and released via the regulated pathway in primary rat cortical neurons.
Wu YJ, Krüttgen A, Möller JC, Shine D, Chan JR, Shooter EM, Cosgaya JM. Wu YJ, et al. J Neurosci Res. 2004 Mar 15;75(6):825-34. doi: 10.1002/jnr.20048. J Neurosci Res. 2004. PMID: 14994343 - Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS.
Lessmann V. Lessmann V. Gen Pharmacol. 1998 Nov;31(5):667-74. doi: 10.1016/s0306-3623(98)00190-6. Gen Pharmacol. 1998. PMID: 9809461 Review. - Brain-Derived Neurotrophic Factor in Suicide Pathophysiology.
Dwivedi Y. Dwivedi Y. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press/Taylor & Francis; 2012. Chapter 8. In: Dwivedi Y, editor. The Neurobiological Basis of Suicide. Boca Raton (FL): CRC Press/Taylor & Francis; 2012. Chapter 8. PMID: 23035300 Free Books & Documents. Review.
Cited by
- Neurotrophin Signaling and Stem Cells-Implications for Neurodegenerative Diseases and Stem Cell Therapy.
Pramanik S, Sulistio YA, Heese K. Pramanik S, et al. Mol Neurobiol. 2017 Nov;54(9):7401-7459. doi: 10.1007/s12035-016-0214-7. Epub 2016 Nov 5. Mol Neurobiol. 2017. PMID: 27815842 Review. - Sorting of internalized neurotrophins into an endocytic transcytosis pathway via the Golgi system: Ultrastructural analysis in retinal ganglion cells.
Butowt R, von Bartheld CS. Butowt R, et al. J Neurosci. 2001 Nov 15;21(22):8915-30. doi: 10.1523/JNEUROSCI.21-22-08915.2001. J Neurosci. 2001. PMID: 11698603 Free PMC article. - ProNGF induces p75-mediated death of oligodendrocytes following spinal cord injury.
Beattie MS, Harrington AW, Lee R, Kim JY, Boyce SL, Longo FM, Bresnahan JC, Hempstead BL, Yoon SO. Beattie MS, et al. Neuron. 2002 Oct 24;36(3):375-86. doi: 10.1016/s0896-6273(02)01005-x. Neuron. 2002. PMID: 12408842 Free PMC article. - Synaptic proteins are tonotopically graded in postnatal and adult type I and type II spiral ganglion neurons.
Flores-Otero J, Davis RL. Flores-Otero J, et al. J Comp Neurol. 2011 Jun 1;519(8):1455-75. doi: 10.1002/cne.22576. J Comp Neurol. 2011. PMID: 21452215 Free PMC article. - BDNF val66met Polymorphism Impairs Hippocampal Long-Term Depression by Down-Regulation of 5-HT3 Receptors.
Hao R, Qi Y, Hou DN, Ji YY, Zheng CY, Li CY, Yung WH, Lu B, Huang Y. Hao R, et al. Front Cell Neurosci. 2017 Oct 12;11:306. doi: 10.3389/fncel.2017.00306. eCollection 2017. Front Cell Neurosci. 2017. PMID: 29075179 Free PMC article.
References
- Acheson A, Barker PA, Alderson RF, Miller FD, Murphy RA. Detection of brain-derived neurotrophic factor-like activity in fibroblasts and Schwann cells: inhibition by antibodies to NGF. Neuron. 1991;7:265–275. - PubMed
- Altar CA, DiStefano PS. Neurotrophin trafficking by anterograde transport. Trends Neurosci. 1998;21:433–437. - PubMed
- Anderson ED, Thomas L, Hayflick JS, Thomas G. Inhibition of HIV-1 gp160-dependent membrane fusion by a furin-directed α1-antitrypsin variant. J Biol Chem. 1993;268:24887–24891. - PubMed
- Arakawa T, Haniu M, Narhi LO, Miller JA, Talvenheimo J, Philo JS, Chute HT, Matheson C, Carnahan J, Louis JC, Yan Q, Welcher AA, Rosenfeld R. Formation of heterodimers from three neurotrophins, nerve growth factor, neurotrophin-3, and brain-derived neurotrophic factor. J Biol Chem. 1994;269:27833–27839. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials