Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain - PubMed (original) (raw)
Efficient particle production by minimal Gag constructs which retain the carboxy-terminal domain of human immunodeficiency virus type 1 capsid-p2 and a late assembly domain
M A Accola et al. J Virol. 2000 Jun.
Abstract
The human immunodeficiency virus type 1 (HIV-1) Gag precursor Pr55(gag) by itself is capable of assembling into retrovirus-like particles (VLP). In the present study, we attempted to identify the minimal Gag sequences required for the formation of VLP. Our results show that about 80% of Pr55(gag) can be either deleted or replaced by heterologous sequences without significantly compromising VLP production. The smallest chimeric molecule still able to efficiently form VLP was only about 16 kDa. This minimal Gag construct contained the leucine zipper domain of the yeast transcription factor GCN4 to substitute for the assembly function of nucleocapsid (NC), followed by a P-P-P-P-Y motif to provide late budding (L) domain function, and retained only the myristylation signal and the C-terminal capsid-p2 domain of Pr55(gag). We also show that the L domain function of HIV-1 p6(gag) is not dependent on the presence of an active viral protease and that the NC domain of Pr55(gag) is dispensable for the incorporation of Vpr into VLP.
Figures
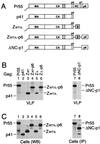
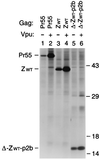
FIG. 1
Replacement of the assembly functions of NC and p6 by GCN4 zipper sequences. (A) Comparison of the domain organizations of the HIV-1 Gag precursor Pr55_gag_ and of mutant Gag molecules. The wavy line at the N termini indicates the presence of a myristylation signal. The position of the MHR within the CA domain is indicated by a cross-hatched box. Horizontal lines denote in-frame deletions, and wild-type or mutant GCN4 zipper (Z) domains inserted in place of Gag sequences are represented by a gray box. (B) VLP formation. HeLa cells were transfected with a PR-defective HIV-1 provirus expressing wild-type Pr55_gag_ or with the indicated Gag mutants, followed by metabolic labeling with [35S]methionine. VLP released during the labeling period were pelleted through sucrose and analyzed by SDS-PAGE. The migration positions of the wild-type and mutant Gag precursors are indicated. (C) Comparison of cell-associated Gag protein levels by Western blotting (WB) with anti-CA antiserum (lanes 1 to 6) or by immunoprecipitation (IP) with patient serum from [35S]methionine-labeled cell lysates (lanes 7 and 8).
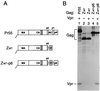
FIG. 2
Vpr incorporation in the absence of NC. (A) Schematic representation of the Gag constructs used. See the legend to Fig. 1 for details. (B) Analysis of Vpr incorporation. HeLa cells were transfected with _vpr_-negative proviral constructs expressing wild-type or mutant Gag precursors. Where indicated, a construct expressing a hemagglutinin epitope-tagged version of HIV-1 Vpr was cotransfected. [35S]methionine-labeled particulate material released into the supernatant was pelleted through sucrose and analyzed directly by SDS-PAGE.
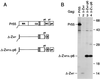
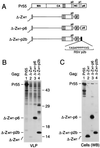
FIG. 3
Efficient VLP formation by minimal HIV-1 Gag constructs. (A) Schematic drawing illustrating the Gag regions retained and the position of the GCN4 zipper domain used to replace the assembly function of NC. See the legend to Fig. 1 for details. (B) VLP formation by transfected HeLa cells. VLP released during metabolic labeling were pelleted through sucrose and analyzed by SDS-PAGE. The positions of wild-type Pr55_gag_ and of chimeric Gag molecules are indicated on the left. The migration positions of molecular mass markers (in kilodaltons) are indicated on the right.
FIG. 4
Comparison of particle densities. [35S]methionine-labeled VLP formed by wild-type Pr55_gag_ and by the chimeric Δ-ZWT-p6 molecule were pooled and fractionated in a 20 to 60% sucrose gradient. Sixteen fractions were collected, precipitated with 10% trichloroacetic acid, and analyzed by SDS-PAGE. The peak Gag protein fractions are shown, and the densities of individual fractions are indicated above each lane. Numbers at right are in kilodaltons.
FIG. 5
Rescue of VLP formation by the L domain of RSV. (A) Schematic presentation of the Gag constructs used. The RSV p2b peptide attached to the C terminus of the wild-type GCN4 leucine zipper (Z) is represented by a black box, and the amino acid sequence of p2b is given in the one-letter code. See the legend to Fig. 1 for other details. (B) VLP formation by transfected HeLa cells. [35S]methionine-labeled particulate material released into the medium was pelleted through sucrose and analyzed directly by SDS-PAGE. Numbers at right are in kilodaltons. (C) Cell-associated Gag detected by Western blotting (WB) with anti-CA antiserum.
FIG. 6
Effect of Vpu on VLP release. HeLa cells were transfected with proviral constructs harboring a wild-type or mutated gag gene and either an intact or a defective vpu gene. Particulate material released during metabolic labeling was pelleted and analyzed directly by SDS-PAGE. Numbers at right are in kilodaltons.
FIG. 7
PR-independent requirement for p6 in the context of a full-length provirus. (A) VLP formation. HeLa cells were transfected with the replication-competent HXBH10 molecular clone (lane 1), with the PR-deficient HXBH10-PR− provirus (lane 4), with a _vpu_-deficient variant of HXBH10 (lane 7), or with versions of HXBH10 and HXBH10-PR− harboring the indicated mutations in p6 (lanes 2, 3, 5, and 6). [35S]methionine-labeled particulate material released by the transfected cells was sedimented through sucrose and analyzed by SDS-PAGE. Numbers at right are in kilodaltons. (B) Cell-associated Gag protein levels determined by immunoprecipitation (IP) from [35S]methionine-labeled cell lysates with patient serum (lanes 1 to 3) or by Western blotting (WB) with anti-CA antiserum (lanes 4 to 7). WT, wild type; Gag PR, Gag precursor.
Similar articles
- Mapping and characterization of the N-terminal I domain of human immunodeficiency virus type 1 Pr55(Gag).
Sandefur S, Smith RM, Varthakavi V, Spearman P. Sandefur S, et al. J Virol. 2000 Aug;74(16):7238-49. doi: 10.1128/jvi.74.16.7238-7249.2000. J Virol. 2000. PMID: 10906178 Free PMC article. - Incorporation of Vpr into human immunodeficiency virus type 1 requires a direct interaction with the p6 domain of the p55 gag precursor.
Bachand F, Yao XJ, Hrimech M, Rougeau N, Cohen EA. Bachand F, et al. J Biol Chem. 1999 Mar 26;274(13):9083-91. doi: 10.1074/jbc.274.13.9083. J Biol Chem. 1999. PMID: 10085158 - Proteolytic activity of human immunodeficiency virus Vpr- and Vpx-protease fusion proteins.
Wu X, Liu H, Xiao H, Kappes JC. Wu X, et al. Virology. 1996 May 1;219(1):307-13. doi: 10.1006/viro.1996.0253. Virology. 1996. PMID: 8623547 - Structure, biological functions and inhibition of the HIV-1 proteins Vpr and NCp7.
Roques BP, Morellet N, de Rocquigny H, Déméné H, Schueler W, Jullian N. Roques BP, et al. Biochimie. 1997 Nov;79(11):673-80. doi: 10.1016/s0300-9084(97)83501-8. Biochimie. 1997. PMID: 9479450 Review. - [Study of molecular function of proteins in human immunodeficiency virus].
Fujita M. Fujita M. Yakugaku Zasshi. 2013;133(10):1103-11. doi: 10.1248/yakushi.13-00200. Yakugaku Zasshi. 2013. PMID: 24088354 Review. Japanese.
Cited by
- cis-Acting determinants of 7SL RNA packaging by HIV-1.
Keene SE, Telesnitsky A. Keene SE, et al. J Virol. 2012 Aug;86(15):7934-42. doi: 10.1128/JVI.00856-12. Epub 2012 May 16. J Virol. 2012. PMID: 22593161 Free PMC article. - The host protein Staufen1 participates in human immunodeficiency virus type 1 assembly in live cells by influencing pr55Gag multimerization.
Chatel-Chaix L, Abrahamyan L, Fréchina C, Mouland AJ, DesGroseillers L. Chatel-Chaix L, et al. J Virol. 2007 Jun;81(12):6216-30. doi: 10.1128/JVI.00284-07. Epub 2007 Apr 11. J Virol. 2007. PMID: 17428849 Free PMC article. - Encapsulating Cas9 into extracellular vesicles by protein myristoylation.
Whitley JA, Kim S, Lou L, Ye C, Alsaidan OA, Sulejmani E, Cai J, Desrochers EG, Beharry Z, Rickman CB, Klingeborn M, Liu Y, Xie ZR, Cai H. Whitley JA, et al. J Extracell Vesicles. 2022 Apr;11(4):e12196. doi: 10.1002/jev2.12196. J Extracell Vesicles. 2022. PMID: 35384352 Free PMC article. - Human immunodeficiency virus type 1 and related primate lentiviruses engage clathrin through Gag-Pol or Gag.
Popov S, Strack B, Sanchez-Merino V, Popova E, Rosin H, Göttlinger HG. Popov S, et al. J Virol. 2011 Apr;85(8):3792-801. doi: 10.1128/JVI.02329-10. Epub 2011 Feb 2. J Virol. 2011. PMID: 21289110 Free PMC article. - HIV-1 gag recruits PACSIN2 to promote virus spreading.
Popov S, Popova E, Inoue M, Wu Y, Göttlinger H. Popov S, et al. Proc Natl Acad Sci U S A. 2018 Jul 3;115(27):7093-7098. doi: 10.1073/pnas.1801849115. Epub 2018 Jun 11. Proc Natl Acad Sci U S A. 2018. PMID: 29891700 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
- R37 AI029873/AI/NIAID NIH HHS/United States
- P30 AI028691/AI/NIAID NIH HHS/United States
- AI29873/AI/NIAID NIH HHS/United States
- AI28691/AI/NIAID NIH HHS/United States
- R01 AI029873/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials