Mapping of atypical protein kinase C within the nerve growth factor signaling cascade: relationship to differentiation and survival of PC12 cells - PubMed (original) (raw)
Mapping of atypical protein kinase C within the nerve growth factor signaling cascade: relationship to differentiation and survival of PC12 cells
M W Wooten et al. Mol Cell Biol. 2000 Jul.
Abstract
The pathway by which atypical protein kinase C (aPKC) contributes to nerve growth factor (NGF) signaling is poorly understood. We previously reported that in PC12 cells NGF-induced activation of mitogen-activated protein kinase (MAPK) occurs independently of classical and nonclassical PKC isoforms, whereas aPKC isoforms were shown to be required for NGF-induced differentiation. NGF-induced activation of PKC-iota was observed to be dependent on phosphatidylinositol 3-kinase (PI3K) and led to coassociation of PKC-iota with Ras and Src. Expression of dominant negative mutants of either Src (DN2) or Ras (Asn-17) impaired activation of PKC-iota by NGF. At the level of Raf-1, neither PKC-iota nor PI3 kinase was required for activation; however, PKC-iota could weakly activate MEK. Inhibitors of PKC-iota activity and PI3K had no effect on NGF-induced MAPK or p38 activation but reduced NGF-stimulated c-Jun N-terminal kinase activity. Src, PI3K, and PKC-iota were likewise required for NGF-induced NF-kappaB activation and cell survival, whereas Ras was not required for either survival or NF-kappaB activation but was required for differentiation. IKK existed as a complex with PKC-iota, Src and IkappaB. Consistent with a role for Src in regulating NF-kappaB activation, an absence of Src activity impaired recruitment of PKC-iota into an IKK complex and markedly impaired NGF-induced translocation of p65/NF-kappaB to the nucleus. These findings reveal that in PC12 cells, aPKCs comprise a molecular switch to regulate differentiation and survival responses coupled downstream to NF-kappaB. On the basis of these findings, Src emerges as a critical upstream regulator of both PKC-iota and the NF-kappaB pathway.
Figures
FIG. 1
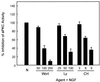
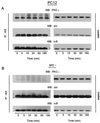
Effects of pharmacological agents on aPKC activity. PC12 cells were pretreated with either wortmannin (Wort; 50, 100, or 200 nM), LY294002 (Ly; 25, 50, or 100 μM), or chelerythrine chloride (CH; 3, 6, or 9 μM) for 1 h prior to addition of NGF (100 ng/ml) for 15 min. PC12 cell lysates (400 μg) were analyzed for aPKC activity in triplicate by immune complex kinase assay using MBP as substrate. Data shown are the means ± standard error of the means of three independent experiments. N, NGF.
FIG. 2
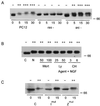
PKC-ι coassociates with Ras and Src. (A) PC12 cells were stimulated with NGF (100 ng/ml) as indicated for 0 to 15 min. Cell lysates (500 μg) were prepared and immunoprecipitated (IP) with antibody to either Ras or Src followed by Western blotting (WB) with PKC-ι antibody. Included in the analysis as a standard was a PC12 cell whole cell lysate (WCL; 70 μg). As positive control, the cell lysates (50 μg) were analyze by immunoblotting with antibody to Ras, Src, or PKC-ι. (B) Control, Ras−, or Src− PC12 cells were stimulated with NGF (100 ng/ml) for 0 to 60 min followed by analysis of aPKC activity in triplicate by immune complex kinase assay using MBP as substrate. This experiment was repeated two other times with similar results.
FIG. 3
Positioning of Ras, Src, and aPKC relative to Raf-1. (A) Control, Ras−, or Src− PC12 cells were stimulated with NGF (100 ng/ml) for 0 to 30 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (7.5% polyacrylamide) and then immunoblotted with Raf-1 antibody. (B) PC12 cells were pretreated with either wortmannin (Wort; 50 or 100 nM), LY294002 (Ly; 25 or 50 μM), or chelerythrine chloride (CH; 3 or 6 μM) for 1 h prior to addition of NGF (100 ng/ml) for 15 min. After lysis, equal protein aliquots were resolved by SDS-PAGE (7.5% polyacrylamide) and then immunoblotted with Raf-1 antibody. C, control; N, NGF. (C) PC12 cells were transfected with control construct, mutant PKC-ι (Imut), or mutant PKC-ζ (Zmut). Thereafter, the cells were stimulated with NGF (100 ng/ml) for 0 or 15 min, as indicated. After lysis, equal protein aliquots were resolved by SDS-PAGE (7.5% polyacrylamide) and then immunoblotted with Raf-1 antibody. Samples were scored + based on the ability of NGF to stimulate Raf-1 hyperphosphorylation/activation as indicated by an increase in relative molecular weight or gel shift (–––). These experiments were repeated two other times with similar results. Bands at 66 kDa are indicated on the right.
FIG. 4
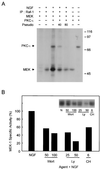
PKC-ι activates MEK. (A) PC12 cells were stimulated with NGF (100 ng/ml) for 15 min followed by immunoprecipitation (IP) of Raf-1 and subjected to an in vitro kinase reaction as indicated with [γ-32P]ATP in the presence of 1 μg of recombinant MEK1. The samples were separated by SDS-PAGE (10% polyacrylamide) followed by autoradiography. Autophosphorylated PKC-ι and phosphorylated MEK1 are shown. Sizes are indicated in kilodaltons. (B) MEK1 activity was measured using immune complex kinase assay as previously described (48). The relative changes in activity was normalized to that obtained with NGF treatment. Similar results were obtained in two other experiments. N, NGF.
FIG. 5
Positioning of Ras, Src, and aPKC relative to MAPK and p38. (A) Control, Ras−, or Src− PC12 cells were stimulated with NGF (100 ng/ml) for 0 to 30 min. After lysis, equal protein aliquots (70 μg) were resolved by SDS-PAGE (12% polyacrylamide) and then immunoblotted with either anti-phospho-MAPK or -p38 antibody. The blots were stripped and reprobed with a nonphospho-specific antibody to MAPK or p38. (B) PC12 cells were pretreated with either wortmannin (Wort; 50 or 100 nM), LY294002 (Ly; 25 or 50 μM), or chelerythrine chloride (CH; 3 or 6 μM) for 1 h prior to addition of NGF (100 ng/ml) for 15 min. After lysis, equal protein aliquots (70 μg) were resolved by SDS-PAGE (12% polyacrylamide) and then immunoblotted with anti-phospho-MAPK or -p38 antibody. This experiment was repeated three times with similar results. C, control; N, NGF.
FIG. 6
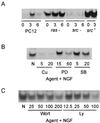
Positioning of Ras, Src, and aPKC relative to NF-κB. (A) PC12, Ras−, Src−, or cells overexpressing c-Src were stimulated with NGF (100 ng/ml) for 0 to 6 h. After lysis, equal protein aliquots were subjected to EMSA analysis. (B) PC12 cells were pretreated with curcumin (Cu; 5 or 20 μM), PD98059 (PD; 15 or 60 μM), or SB202190 (SB; 5 or 20 μM) followed by addition of NGF (100 ng/ml) for 3 h. After lysis, equal protein aliquots were subjected to EMSA analysis. (C) PC12 cells were pretreated with either wortmannin (Wort; 25 to 200 nM) or LY294002 (Ly; 12.5 to 100 μM) for 1 h prior to addition of NGF (100 ng/ml) for 3 h. After lysis, equal protein aliquots were subjected to EMSA analysis. This experiment was repeated twice with similar results.
FIG. 7
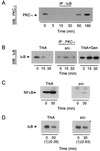
Src regulates the association of IKK with aPKC. PC12 cells, parental or Src−, were treated with NGF (100 ng/ml) for the times indicated, and lysates (500 μg) were immunoprecipitated with anti-IKK. The immunoprecipitates (IP:IKK) or lysates (50 μg), as a positive control, were Western blotted (WB) with PKC-ι, Src, and IκB antibodies. This experiment was repeated twice with similar results.
FIG. 8
Src is required for PKC-ι/IκB coassociation. (A) PC12 cells were treated with NGF (100 ng/ml) for the times indicated, and lysates were immunoprecipitated (IP) with anti-IκB. The precipitates were Western blotted (WB) with PKC-ι antibody. (B) PC12 cells, cells pretreated with genistein (Gen; 20 μM), or Src− cells were treated with NGF (100 ng/ml) for the times indicated, and lysates were immunoprecipitated with anti-PKC-ι. The precipitates were blotted with IκBα. (C) PC12 or Src− cells were exposed to NGF (100 ng/ml) for 30 min. Nuclei were isolated and immunoblotted with anti-p65/NF-κB antibody. (D) PC12 or Src− cells were exposed to NGF (100 ng/ml) for 30 min. Whole cell lysates were prepared (50 μg) and immunoblotted with antibody to IκB. The blots were scanned, and the relative change in intensity of IκB is shown in parentheses.
FIG. 9
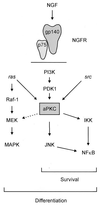
Model illustrating the relative positioning of aPKC within the NGF signaling cascades for survival and differentiation. aPKC can activate MEK and lies upstream of JNK. Both Ras and Src lie upstream of aPKC, as does PI3K, whereas aPKC lies upstream of NF-κB, directly interacting with IKK. Differentiation requires components of both the Src and Ras pathways. The differentiation pathway overlaps with components of the survival pathway through Src, aPKC, and PI3K. Survival signaling is Ras independent but dependent on PI3K, Src, aPKC, and NF-κB. Our findings support a model whereby aPKC occupies a critical node, capable of interacting with Ras-MAPK through modulation of MEK and JNK and upstream of NF-κB. NGFR, NGF receptor.
Similar articles
- Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C's via a src kinase pathway.
Wooten MW, Vandenplas ML, Seibenhener ML, Geetha T, Diaz-Meco MT. Wooten MW, et al. Mol Cell Biol. 2001 Dec;21(24):8414-27. doi: 10.1128/MCB.21.24.8414-8427.2001. Mol Cell Biol. 2001. PMID: 11713277 Free PMC article. - Overexpression of atypical PKC in PC12 cells enhances NGF-responsiveness and survival through an NF-kappaB dependent pathway.
Wooten MW, Seibenhener ML, Zhou G, Vandenplas ML, Tan TH. Wooten MW, et al. Cell Death Differ. 1999 Aug;6(8):753-64. doi: 10.1038/sj.cdd.4400548. Cell Death Differ. 1999. PMID: 10467349 - The atypical PKCs in inflammation: NF-κB and beyond.
Diaz-Meco MT, Moscat J. Diaz-Meco MT, et al. Immunol Rev. 2012 Mar;246(1):154-67. doi: 10.1111/j.1600-065X.2012.01093.x. Immunol Rev. 2012. PMID: 22435553 Free PMC article. Review. - Sequestosome1/p62: a regulator of redox-sensitive voltage-activated potassium channels, arterial remodeling, inflammation, and neurite outgrowth.
Ishii T, Warabi E, Siow RCM, Mann GE. Ishii T, et al. Free Radic Biol Med. 2013 Dec;65:102-116. doi: 10.1016/j.freeradbiomed.2013.06.019. Epub 2013 Jun 19. Free Radic Biol Med. 2013. PMID: 23792273 Review.
Cited by
- Altered expression of atypical PKC and Ryk in the spinal cord of a mouse model of amyotrophic lateral sclerosis.
Tury A, Tolentino K, Zou Y. Tury A, et al. Dev Neurobiol. 2014 Aug;74(8):839-50. doi: 10.1002/dneu.22137. Epub 2014 Jan 22. Dev Neurobiol. 2014. PMID: 24123880 Free PMC article. Review. - Atypical protein kinase Cλ is critical for growth factor receptor-induced dorsal ruffle turnover and cell migration.
Xing B, Wang L, Guo D, Huang J, Espenel C, Kreitzer G, Zhang JJ, Guo L, Huang XY. Xing B, et al. J Biol Chem. 2013 Nov 15;288(46):32827-36. doi: 10.1074/jbc.M113.489427. Epub 2013 Oct 3. J Biol Chem. 2013. PMID: 24092753 Free PMC article. - Nerve growth factor stimulates multisite tyrosine phosphorylation and activation of the atypical protein kinase C's via a src kinase pathway.
Wooten MW, Vandenplas ML, Seibenhener ML, Geetha T, Diaz-Meco MT. Wooten MW, et al. Mol Cell Biol. 2001 Dec;21(24):8414-27. doi: 10.1128/MCB.21.24.8414-8427.2001. Mol Cell Biol. 2001. PMID: 11713277 Free PMC article. - The basic region and leucine zipper transcription factor MafK is a new nerve growth factor-responsive immediate early gene that regulates neurite outgrowth.
Töröcsik B, Angelastro JM, Greene LA. Töröcsik B, et al. J Neurosci. 2002 Oct 15;22(20):8971-80. doi: 10.1523/JNEUROSCI.22-20-08971.2002. J Neurosci. 2002. PMID: 12388604 Free PMC article. - IkappaB kinase beta phosphorylates Dok1 serines in response to TNF, IL-1, or gamma radiation.
Lee S, Andrieu C, Saltel F, Destaing O, Auclair J, Pouchkine V, Michelon J, Salaun B, Kobayashi R, Jurdic P, Kieff ED, Sylla BS. Lee S, et al. Proc Natl Acad Sci U S A. 2004 Dec 14;101(50):17416-21. doi: 10.1073/pnas.0408061101. Epub 2004 Dec 1. Proc Natl Acad Sci U S A. 2004. PMID: 15574499 Free PMC article.
References
- Abu-Amer Y, Ross F P, McHugh K P, Livolsi A, Peyron J, Teitelbaum S L. Tumor necrosis factor-α activation of nuclear transcription factor-κB in marrow macrophages is mediated by c-Src tyrosine phosphorylation of IκBα. J Biol Chem. 1998;273:29417–29423. - PubMed
- Bar-Sagi D, Feramisco J R. Microinjection of the ras oncogene protein into PC12 cells induced morphological differentiation. Cell. 1985;42:841–848. - PubMed
- Berra E, Diaz-Meco M T, Dominguez I, Municio M M, Sanz L, Lozano J, Chapkin R S, Moscat J. Protein kinase C ζ isoform is critical for mitogenic signal transduction. Cell. 1993;74:555–563. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous