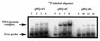Substitution of Asp-210 in HAP1 (APE/Ref-1) eliminates endonuclease activity but stabilises substrate binding - PubMed (original) (raw)
Substitution of Asp-210 in HAP1 (APE/Ref-1) eliminates endonuclease activity but stabilises substrate binding
D G Rothwell et al. Nucleic Acids Res. 2000.
Abstract
HAP1, also known as APE/Ref-1, is the major apurinic/apyrimidinic (AP) endonuclease in human cells. Previous structural studies have suggested a possible role for the Asp-210 residue of HAP1 in the enzymatic function of this enzyme. Here, we demonstrate that substitution of Asp-210 by Asn or Ala eliminates the AP endonuclease activity of HAP1, while substitution by Glu reduces specific activity approximately 500-fold. Nevertheless, these mutant proteins still bind efficiently to oligonucleotides containing either AP sites or the chemically unrelated bulky p-benzoquinone (pBQ) derivatives of dC, dA and dG, all of which are substrates for HAP1. These results indicate that Asp-210 is required for catalysis, but not substrate recognition, consistent with enzyme kinetic data indicating that the HAP1-D210E protein has a 3000-fold reduced K(cat )for AP site cleavage, but an unchanged K(m). Through analysis of the binding of Asp-210 substitution mutants to oligonucleotides containing either an AP site or a pBQ adduct, we conclude that the absence of Asp-210 allows the formation of a stable HAP1-substrate complex that exists only transiently during the catalytic cycle of wild-type HAP1 protein. We interpret these data in the context of the structure of the HAP1 active site and the recently determined co-crystal structure of HAP1 bound to DNA substrates.
Figures
Figure 1
A conserved motif in the exonuclease III family of AP endonucleases. The amino acid sequence of HAP1 has been aligned with those of AP endonucleases from different species: bovine (BAP1), murine (APEX), Arabidopsis thaliana (Arp1), Drosophila melanogaster (Rrp1), Streptococcus pneumoniae (ExoA) and E.coli (exonuclease III). The conserved residues, Asp-210 and Asn-212 in HAP1, are shown in bold type with an asterisk below.
Figure 2
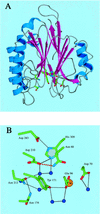
(A) Overall view of HAP1 (NDB/PDB accession no. 1BIX). α helices are shown in blue, and β strands are shown in magenta. Active site residues are represented as atom coloured sticks. (B) Close-up view of active site residues. Side chains are represented as atom coloured sticks, water molecules are shown as blue spheres, the bound metal ion is shown as a gold sphere. Hydrogen bonds are represented by black dots. The hydrogen bond between Asp-210 and Asn-68 is shown in red. For clarity, hydrogen bonds to main chain atoms have been omitted.
Figure 3
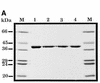
(A) Purification of site-specific mutant HAPI proteins with substitutions at position 210. A Coomassie blue-stained SDS–polyacrylamide gel is shown. Lane 1, wild-type HAP1; lane 2, HAP1–D210E; lane 3, HAP1–D210N; lane 4, HAP1–D210A. M, molecular mass standards, as indicated on the left. HAP1 has an apparent mass of 37 kDa. (B) Substitution of Asp-210 does not influence the ‘redox’ activity of HAP1. Oxidised Jun protein (100 nM) was incubated with 25 mM dithiothreitol (positive control, lane 1), or with 500 nM wild-type HAP1 (lane 2), HAP1–D210E (lane 3), HAP1–D210N (lane 4), HAP1–D210A (lane 5) or no additional factors (lane 6), and a band-shift assay was performed using a 32P-labelled oligonucleotide containing a consensus Jun binding site. The positions of the free oligonucleotide and the Jun–DNA complex are indicated on the left.
Figure 3
(A) Purification of site-specific mutant HAPI proteins with substitutions at position 210. A Coomassie blue-stained SDS–polyacrylamide gel is shown. Lane 1, wild-type HAP1; lane 2, HAP1–D210E; lane 3, HAP1–D210N; lane 4, HAP1–D210A. M, molecular mass standards, as indicated on the left. HAP1 has an apparent mass of 37 kDa. (B) Substitution of Asp-210 does not influence the ‘redox’ activity of HAP1. Oxidised Jun protein (100 nM) was incubated with 25 mM dithiothreitol (positive control, lane 1), or with 500 nM wild-type HAP1 (lane 2), HAP1–D210E (lane 3), HAP1–D210N (lane 4), HAP1–D210A (lane 5) or no additional factors (lane 6), and a band-shift assay was performed using a 32P-labelled oligonucleotide containing a consensus Jun binding site. The positions of the free oligonucleotide and the Jun–DNA complex are indicated on the left.
Figure 4
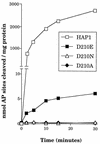
Effects of substitutions at position 210 on the AP endonuclease activity of HAP1. Data represent the rates of cleavage of an oligonucleotide containing a single AP site catalysed by wild-type HAP1 (open squares), HAP1–D210E (closed squares), HAP1–D210N (open triangles) and HAP1–D210A (closed diamonds). Note that the vertical axis is broken.
Figure 5
(A) A band-shift assay to show that substitution of residue 210 in HAP1 does not eliminate AP site-binding. 500 nM HAP1 (lane 1), HAP1–D210E (lane 2), HAP1–D210N (lane 3) or HAP1–D210A (lane 4) proteins were incubated in the presence of 4 mM EDTA with 0.5 ng 32P-labelled oligonucleotide containing a single AP site, and the products were separated on a polyacrylamide–TBE gel. In lane 5, the reaction mixture contained no HAP1 protein. The positions of the free oligonucleotide and the HAP1–DNA complex are shown on the right. (B) Binding of HAP1–D210N protein to a pBQ-dC-containing oligonucleotide in the presence of 5 mM EDTA (lanes 1–5) or in the absence of EDTA (lanes 6–10). Lanes 1 and 6, no HAP1; lanes 2 and 7, 62.5 ng HAP1–D210N; lanes 3 and 8, 125 ng HAP1–D210N; lanes 4 and 9, 250 ng HAP1–D210N; lanes 5 and 10, 500 ng HAP1–D210N. Lane 11 shows the position of the free probe. (C) Effect of substitution at position 210 of HAP1 on the rate of cleavage of a pBQ-dC-containing oligonucleotide. The time course of cleavage for wild-type HAP1 (squares) and HAP1–D210N (triangles) was measured using 180 ng protein, as described in Materials and Methods.
Figure 5
(A) A band-shift assay to show that substitution of residue 210 in HAP1 does not eliminate AP site-binding. 500 nM HAP1 (lane 1), HAP1–D210E (lane 2), HAP1–D210N (lane 3) or HAP1–D210A (lane 4) proteins were incubated in the presence of 4 mM EDTA with 0.5 ng 32P-labelled oligonucleotide containing a single AP site, and the products were separated on a polyacrylamide–TBE gel. In lane 5, the reaction mixture contained no HAP1 protein. The positions of the free oligonucleotide and the HAP1–DNA complex are shown on the right. (B) Binding of HAP1–D210N protein to a pBQ-dC-containing oligonucleotide in the presence of 5 mM EDTA (lanes 1–5) or in the absence of EDTA (lanes 6–10). Lanes 1 and 6, no HAP1; lanes 2 and 7, 62.5 ng HAP1–D210N; lanes 3 and 8, 125 ng HAP1–D210N; lanes 4 and 9, 250 ng HAP1–D210N; lanes 5 and 10, 500 ng HAP1–D210N. Lane 11 shows the position of the free probe. (C) Effect of substitution at position 210 of HAP1 on the rate of cleavage of a pBQ-dC-containing oligonucleotide. The time course of cleavage for wild-type HAP1 (squares) and HAP1–D210N (triangles) was measured using 180 ng protein, as described in Materials and Methods.
Figure 5
(A) A band-shift assay to show that substitution of residue 210 in HAP1 does not eliminate AP site-binding. 500 nM HAP1 (lane 1), HAP1–D210E (lane 2), HAP1–D210N (lane 3) or HAP1–D210A (lane 4) proteins were incubated in the presence of 4 mM EDTA with 0.5 ng 32P-labelled oligonucleotide containing a single AP site, and the products were separated on a polyacrylamide–TBE gel. In lane 5, the reaction mixture contained no HAP1 protein. The positions of the free oligonucleotide and the HAP1–DNA complex are shown on the right. (B) Binding of HAP1–D210N protein to a pBQ-dC-containing oligonucleotide in the presence of 5 mM EDTA (lanes 1–5) or in the absence of EDTA (lanes 6–10). Lanes 1 and 6, no HAP1; lanes 2 and 7, 62.5 ng HAP1–D210N; lanes 3 and 8, 125 ng HAP1–D210N; lanes 4 and 9, 250 ng HAP1–D210N; lanes 5 and 10, 500 ng HAP1–D210N. Lane 11 shows the position of the free probe. (C) Effect of substitution at position 210 of HAP1 on the rate of cleavage of a pBQ-dC-containing oligonucleotide. The time course of cleavage for wild-type HAP1 (squares) and HAP1–D210N (triangles) was measured using 180 ng protein, as described in Materials and Methods.
Figure 6
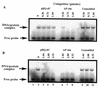
Specificity of HAP1–D210N protein binding to oligonucleotide duplex containing the pBQ-dC adduct. HAP1–D210N protein (34 nM) was incubated with 32P-end-labelled 25mer pBQ-dC oligonucleotide and a molar excess of oligonucleotide competitors (AP site-containing, pBQ-dC or unmodified; as indicated above the lanes). (A) Low range concentrations of competitors (20-, 40- and 80-fold molar excess) were used, while in (B), higher concentrations (80-, 160- and 240-fold molar excess) of the same competitors were used. Lane 1 in each panel contained binding buffer only, and lane 2 contained HAP1–D210N protein, but without any competitor added. All reactions contained 5 mM EDTA.
Figure 7
Differential binding of D210N to oligonucleotides with pBQ-dA, pBQ-dC or pBQ-dG adduct. All three pBQ adduct-containing 25mers were 32P-end-labelled and annealed to a common complementary strand (Materials and Methods). Binding reactions were carried out with increasing amounts of D210N protein in the presence of 5 mM EDTA. Lanes 1, 5 and 9, controls with binding buffer only. Lanes 2, 6 and 10 contained 125 ng HAP1–D210N; lanes 3, 7 and 11 contained 250 ng HAP1–D210N; lanes 4, 8 and 12 contained 500 ng HAP1–D210N. Arrows are as indicated in Figure 6.
Similar articles
- Evidence for a common active site for cleavage of an AP site and the benzene-derived exocyclic adduct, 3,N4-benzetheno-dC, in the major human AP endonuclease.
Hang B, Rothwell DG, Sagi J, Hickson ID, Singer B. Hang B, et al. Biochemistry. 1997 Dec 9;36(49):15411-8. doi: 10.1021/bi971367s. Biochemistry. 1997. PMID: 9398271 - Elements in abasic site recognition by the major human and Escherichia coli apurinic/apyrimidinic endonucleases.
Erzberger JP, Barsky D, Schärer OD, Colvin ME, Wilson DM 3rd. Erzberger JP, et al. Nucleic Acids Res. 1998 Jun 1;26(11):2771-8. doi: 10.1093/nar/26.11.2771. Nucleic Acids Res. 1998. PMID: 9592167 Free PMC article. - What structural features determine repair enzyme specificity and mechanism in chemically modified DNA?
Singer B, Hang B. Singer B, et al. Chem Res Toxicol. 1997 Jul;10(7):713-32. doi: 10.1021/tx970011e. Chem Res Toxicol. 1997. PMID: 9250405 Review. - The structure and functions of the HAP1/Ref-1 protein.
Rothwell DG, Barzilay G, Gorman M, Morera S, Freemont P, Hickson ID. Rothwell DG, et al. Oncol Res. 1997;9(6-7):275-80. Oncol Res. 1997. PMID: 9406232 Review.
Cited by
- Mutational and Kinetic Analysis of APE1 Endoribonuclease Activity.
Kuznetsova AA, Gavrilova AA, Novopashina DS, Fedorova OS, Kuznetsov NA. Kuznetsova AA, et al. Mol Biol. 2021;55(2):211-224. doi: 10.1134/S0026893321020102. Epub 2021 Apr 29. Mol Biol. 2021. PMID: 33948042 Free PMC article. - Structural and Functional Characterization of a Unique AP Endonuclease From Deinococcus radiodurans.
He Y, Wang Y, Qin C, Xu Y, Cheng K, Xu H, Tian B, Zhao Y, Wang L, Hua Y. He Y, et al. Front Microbiol. 2020 Jun 5;11:1178. doi: 10.3389/fmicb.2020.01178. eCollection 2020. Front Microbiol. 2020. PMID: 33117296 Free PMC article. - A panel of colorimetric assays to measure enzymatic activity in the base excision DNA repair pathway.
Healing E, Charlier CF, Meira LB, Elliott RM. Healing E, et al. Nucleic Acids Res. 2019 Jun 20;47(11):e61. doi: 10.1093/nar/gkz171. Nucleic Acids Res. 2019. PMID: 30869144 Free PMC article. - Structural basis for recognition and repair of the 3'-phosphate by NExo, a base excision DNA repair nuclease from Neisseria meningitidis.
Silhan J, Zhao Q, Boura E, Thomson H, Förster A, Tang CM, Freemont PS, Baldwin GS. Silhan J, et al. Nucleic Acids Res. 2018 Dec 14;46(22):11980-11989. doi: 10.1093/nar/gky934. Nucleic Acids Res. 2018. PMID: 30329088 Free PMC article. - High-resolution crystal structures reveal plasticity in the metal binding site of apurinic/apyrimidinic endonuclease I.
He H, Chen Q, Georgiadis MM. He H, et al. Biochemistry. 2014 Oct 21;53(41):6520-9. doi: 10.1021/bi500676p. Epub 2014 Oct 8. Biochemistry. 2014. PMID: 25251148 Free PMC article.
References
- Doetsch P.W. and Cunningham,R.P. (1990) Mutat. Res., 236, 173–201. - PubMed
- Lindahl T. (1990) Mutat. Res., 238, 305–311. - PubMed
- Lindahl T. (1993) Nature, 362, 709–715. - PubMed
- Barzilay G. and Hickson,I.D. (1995) Bioessays, 17, 713–719. - PubMed
- Wallace S.S. (1998) Radiat. Res., 150, S60–S79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous