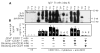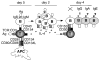Engagement of CD153 (CD30 ligand) by CD30+ T cells inhibits class switch DNA recombination and antibody production in human IgD+ IgM+ B cells - PubMed (original) (raw)
Engagement of CD153 (CD30 ligand) by CD30+ T cells inhibits class switch DNA recombination and antibody production in human IgD+ IgM+ B cells
A Cerutti et al. J Immunol. 2000.
Abstract
CD153 (CD30 ligand) is a member of the TNF ligand/cytokine family expressed on the surface of human B cells. Upon exposure to IL-4, a critical Ig class switch-inducing cytokine, Ag-activated T cells express CD30, the CD153 receptor. The observation that dysregulated IgG, IgA, and/or IgE production is often associated with up-regulation of T cell CD30 prompted us to test the hypothesis that engagement of B cell CD153 by T cell CD30 modulates Ig class switching. In this study, we show that IgD+ IgM+ B cells up-regulate CD153 in the presence of CD154 (CD40 ligand), IL-4, and B cell Ag receptor engagement. In these cells, CD153 engagement by an agonistic anti-CD153 mAb or T cell CD30 inhibits S mu-->Sgamma, Smu-->Salpha, and S mu-->Sepsilon class switch DNA recombination (CSR). This inhibition is associated with decreased TNFR-associated factor-2 binding to CD40, decreased NF-kappaB binding to the CD40-responsive element of the Cgamma3 promoter, decreased Igamma3-Cgamma3 germline gene transcription, and decreased expression of Ku70, Ku80, DNA protein kinase, switch-associated protein-70, and Msh2 CSR-associated transcripts. In addition, CD153 engagement inhibits IgG, IgA, and IgE production, and this effect is associated with reduced levels of B lymphocyte maturation protein-1 transcripts, and increased binding of B cell-specific activation protein to the Ig 3' enhancer. These findings suggest that CD30+ T cells modulate CSR as well as IgG, IgA, and IgE production by inducing reverse signaling through B cell CD153.
Figures
FIGURE 1
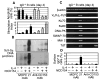
Human B cells up-regulate CD153 in the GC of secondary lymphoid organs. A, CD153 expression was analyzed by flow cytometry on electronically gated IgD+ CD38− (B1, naive), IgD+ CD38+ (B2, founder GC), IgD− CD38+ (B3, GC), and CD38− IgD− (B4, memory) B cells. Filled and shaded histograms correspond to control and anti-CD153 mAbs, respectively. β_-actin (593-bp), I_γ_3-C_γ_3 (670-bp), and VDJ-C_γ_3 (416-bp) transcripts were PCR amplified (35 cycles) from each B cell fraction. B, IgD, CD153, and CD79a (Ig_α) were visualized (brown cells) in serial sections from the same lymphoid follicle by immunohistochemistry (×400). C, Expression of CD153 was analyzed on freshly isolated tonsil IgD+ B cells (day 0) or on IgD+ B cells exposed to htCD154 and IL-4 with or without anti-BCR Abs.
FIGURE 2
CD153 cross-linking inhibits germline I_γ_3-C_γ_3 transcription in CD154- and IL-4-induced IgD+ B cells. PB IgD+ B cells were cultured for 2 days with or without htCD154 and IL-4, and in the presence or absence of MOPC-21 mAb, anti-CD44 mAb, anti-CD134L mAb, anti-CD153 mAb, human CD192:Fc IgG1, or human CD30:Fc IgG1 immobilized on irradiated CD32 L cells. _β_-actin (593-bp) and I_γ_3-C_γ_3 (670-bp) transcripts were PCR amplified (18 cycles) from equal amounts of cDNA in the presence of [_α_-32P]dCTP. PCR products were then fractionated on a 6% polyacrylamide gel. These findings were derived from one of three experiments yielding comparable results.
FIGURE 3
CD153 cross-linking inhibits S_μ_ →S_γ_3 CSR in CD154-and IL-4-induced IgD+ B cells. PB IgD+ B cells were cultured for 4 days with or without htCD154 and/or IL-4, and in the presence or absence of MOPC-21 or anti-CD153 mAbs immobilized on CD32 L cells. A, B cell apoptosis and proliferation measured by propidium iodide plus annexin V staining and [3H]TdR incorporation assays, respectively. B, S_γ_3-S_μ_ DNAs (0.5–3 kb) PCR amplified (30 cycles) from 500 ng of genomic DNA, and hybridized with S_μ_ and S_γ_ probes. C, _β_-actin (593 bp), VHDJH-C_γ_3 (416 bp), Ku70 (518 bp), Ku80 (492 bp), DNA-PK (578 bp), SWAP-70 (548 bp), Msh2 (449 bp), and TdT (276 bp) PCR amplified (25 cycles; _β_-actin 20 cycles) from equal amounts of cDNAs. D, Percentage of CD19+ IgD+ B cells expressing surface IgG (arrows indicate <0.05). These findings were derived from one of five experiments yielding comparable results (error bars indicate ± SD).
FIGURE 4
CD153 cross-linking inhibits activation of the C_γ_3 germline gene promoter, nuclear translocation of NF-_κ_B, and CD40:TRAF-2 association in CD154- and IL-4-induced IgD+ B cells. CL-01 B cells were cultured with or without htCD154 and/or IL-4, and in the presence of MOPC-21 or anti-CD153 mAbs immobilized on the plastic plate. A, _β_-actin (593-bp), RAG-2 (1,105 bp), and I_γ_3-C_γ_3 (670-bp) transcripts were PCR amplified (25 cycles) from equal amounts of cDNA. B, CL-01 cells were transfected with an ECS-I_γ_3-pGL3 construct, and the luciferase activity was measured after 24 h. C, The binding of NF-_κ_B and STAT-6 to the CD40 and IL-4R REs of the C_γ_3 promoter was assessed by EMSA. D, Cytoplasmic proteins obtained from 4-h-stimulated B cells were transferred to nitrocellulose membranes, and immunoblotted for TRAF-2 and actin. In additional experiments, total proteins immunoprecipitated with an anti-CD40 mAb were immunoblotted for TRAF-2. These findings were derived from one of three experiments yielding comparable results (error bars indicate ± SD).
FIGURE 5
CD153 cross-linking down-regulates IgG secretion and Blimp-1 transcripts while up-regulating BSAP nuclear activity in CD154- and cytokine-induced IgD+ B cells. A, Tonsil IgD+ (■) or IgG+ (□) B cells were cultured with or without htCD154 and cytokines (IL-4 and IL-10), and in the presence of MOPC-21 or anti-CD153 mAbs immobilized on CD32 L cells. IgG concentration and CD38++ CD138+ plasmacytoid B cells were analyzed after 8 days (arrows indicate IgG and plasma cell values <100 ng/ml and <0.1%, respectively). B, Tonsil IgD+ B cells were cultured as above. After 4 days, IL-6 was measured by ELISA; Blimp-1 (355-bp) and _β_-actin (593-bp) transcripts were PCR amplified (25 cycles) from equal amounts of cDNA; and BSAP binding to the BSAP site 1 of the mouse Ig 3′ enhancer was determined by EMSA. These findings were derived from one of three experiments yielding comparable results (error bars indicate ± SD).
FIGURE 6
CD4+ T cells express CD154 and CD30 4 days after TCR, CD28, and CD134 stimulation. A and B, PBMCs were incubated with IL-4 and mAbs to CD3, CD28, and CD134 immobilized on CD32 L cells. After 8 h (A) or 4 days (B), CD4, CD154, and CD30 were analyzed on gated CD3+ T cells. C, IgD+ (■) or IgG+ (□) B cells were cultured with or without cytokines (IL-4 and IL-10), anti-BCR Abs, and CD4+ T cells isolated from 4-day-stimulated PBMCs. Before culture, CD4+ T cells were fixed and preincubated with MOPC-21 or blocking mAbs to CD154, CD27, or CD30. IgG were measured after 8 days. These findings were derived from one of three experiments yielding comparable results (error bars indicate ± SD).
FIGURE 7
CD30+ T cells inhibit CSR in CD154- and cytokine-induced IgD+ B cells. IgD+ B cells were cultured with htCD154, cytokines (IL-4 and IL-10), and anti-BCR Abs in the presence or absence of CD4+ CD30− or CD4+ CD30+ T cells sorted from 4-day-stimulated PBMCs. Before culture, all CD4+ T cells were fixed, washed, incubated with a blocking anti-CD154 mAb, and washed. CD30+ T cells were also preincubated with blocking anti-CD30 or anti-CD27 mAbs. S_γ_-S_μ_ (γ), S_α_-S_μ_ (α), and S_ε_-S_μ_ (ε) junction DNAs (A) as well as IgG, IgA, and IgE secretion (B) were assessed after 8 days. These findings were derived from one of three experiments yielding comparable results (error bars indicate ± SD).
FIGURE 8
Proposed role of CD30+ T cells in the regulation of CSR and IgG, IgA, and IgE production. Upon TCR, CD28, and CD134 engagement by Ag and costimulatory molecules on APCs, CD4+ Th cells express CD154 and secrete IL-4. In the presence of Ag, CD154, and IL-4, IgM+ IgD+ CD30− B cells undergo clonal expansion and up-regulate CD153. Within 4 days, Ag-selected B cells complete CSR to IgG, IgA, or IgE and down-regulate CD153. At this time, CD4+ T cells induce CD30 while still expressing significant levels of CD154. These CD30+ T cells inhibit CSR in newly CD154:CD40-activated IgD+ and/or IgM+ B cells by inducing reverse signaling through CD153.
Similar articles
- EBV-encoded latent membrane protein 1 cooperates with BAFF/BLyS and APRIL to induce T cell-independent Ig heavy chain class switching.
He B, Raab-Traub N, Casali P, Cerutti A. He B, et al. J Immunol. 2003 Nov 15;171(10):5215-24. doi: 10.4049/jimmunol.171.10.5215. J Immunol. 2003. PMID: 14607922 Free PMC article. - Dysregulation of CD30+ T cells by leukemia impairs isotype switching in normal B cells.
Cerutti A, Kim EC, Shah S, Schattner EJ, Zan H, Schaffer A, Casali P. Cerutti A, et al. Nat Immunol. 2001 Feb;2(2):150-6. doi: 10.1038/84254. Nat Immunol. 2001. PMID: 11175813 Free PMC article. - A family of ligands for the TNF receptor superfamily.
Cosman D. Cosman D. Stem Cells. 1994 Sep;12(5):440-55. doi: 10.1002/stem.5530120501. Stem Cells. 1994. PMID: 7528588 Review. - Regulation of the IgE isotype switch: new insights on cytokine signals and the functions of epsilon germline transcripts.
Oettgen HC. Oettgen HC. Curr Opin Immunol. 2000 Dec;12(6):618-23. doi: 10.1016/s0952-7915(00)00153-9. Curr Opin Immunol. 2000. PMID: 11102763 Review.
Cited by
- CD30 on extracellular vesicles from malignant Hodgkin cells supports damaging of CD30 ligand-expressing bystander cells with Brentuximab-Vedotin, in vitro.
Hansen HP, Trad A, Dams M, Zigrino P, Moss M, Tator M, Schön G, Grenzi PC, Bachurski D, Aquino B, Dürkop H, Reiners KS, von Bergwelt-Baildon M, Hallek M, Grötzinger J, Engert A, Paes Leme AF, Pogge von Strandmann E. Hansen HP, et al. Oncotarget. 2016 May 24;7(21):30523-35. doi: 10.18632/oncotarget.8864. Oncotarget. 2016. PMID: 27105521 Free PMC article. - Immunoglobulin somatic hypermutation: double-strand DNA breaks, AID and error-prone DNA repair.
Wu X, Feng J, Komori A, Kim EC, Zan H, Casali P. Wu X, et al. J Clin Immunol. 2003 Jul;23(4):235-46. doi: 10.1023/a:1024571714867. J Clin Immunol. 2003. PMID: 12959216 Free PMC article. Review. - Deciphering CD30 ligand biology and its role in humoral immunity.
Kennedy MK, Willis CR, Armitage RJ. Kennedy MK, et al. Immunology. 2006 Jun;118(2):143-52. doi: 10.1111/j.1365-2567.2006.02354.x. Immunology. 2006. PMID: 16771849 Free PMC article. Review. - Mast cell CD30 ligand is upregulated in cutaneous inflammation and mediates degranulation-independent chemokine secretion.
Fischer M, Harvima IT, Carvalho RF, Möller C, Naukkarinen A, Enblad G, Nilsson G. Fischer M, et al. J Clin Invest. 2006 Oct;116(10):2748-56. doi: 10.1172/JCI24274. Epub 2006 Sep 7. J Clin Invest. 2006. PMID: 16964309 Free PMC article. - IL-21 conditions antigen-presenting human γδ T-cells to promote IL-10 expression in naïve and memory CD4+ T-cells.
Tyler CJ, Hoti I, Griffiths DD, Cuff SM, Andrews R, Keisker M, Ahmed R, Hansen HP, Lindsay JO, Stagg AJ, Moser B, McCarthy NE, Eberl M. Tyler CJ, et al. Discov Immunol. 2024 May 13;3(1):kyae008. doi: 10.1093/discim/kyae008. eCollection 2024. Discov Immunol. 2024. PMID: 38903247 Free PMC article.
References
- Fujieda S, Zhang K, Saxon A. IL-4 plus CD40 monoclonal antibody induces human B cell γ subclass-specific isotype switch: switching to γ1, γ3, and γ4, but not γ2. J Immunol. 1995;155:2318. - PubMed
- Cerutti A, Zan H, Schaffer A, Bergsagel L, Harindranath N, Max EE, Casali P. CD40 ligand and appropriate cytokines induce switching to IgG, IgA, and IgE and coordinated germinal center-like phenotype differentiation in a human monoclonal IgM+ IgD+ B cell line. J Immunol. 1998;160:2145. - PMC - PubMed
- Zan H, Cerutti A, Schaffer A, Dramitinos P, Casali P. CD40 engagement triggers switching to IgA1 and IgA2 in human B cells through induction of endogenous TGF-β: evidence for TGF-β-dependent but not IL-10-dependent direct Sμ→Sα and sequential Sμ→Sγ, Sγ→Sα DNA recombination. J Immunol. 1998;162:5217. - PMC - PubMed
- Van Kooten C, Banchereau J. CD40-CD40 ligand: a multifunctional receptor-ligand pair. Adv Immunol. 1996;61:1. - PubMed
- Grewal IS, Flavell RA. CD40 and CD154 in cell-mediated immunity. Annu Rev Immunol. 1998;16:111. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AR40908/AR/NIAMS NIH HHS/United States
- R01 AR040908/AR/NIAMS NIH HHS/United States
- R56 AI045011/AI/NIAID NIH HHS/United States
- AG 13910/AG/NIA NIH HHS/United States
- R01 AI045011/AI/NIAID NIH HHS/United States
- AI 45011/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous