Activation of p53 in cervical carcinoma cells by small molecules - PubMed (original) (raw)
Activation of p53 in cervical carcinoma cells by small molecules
S Hietanen et al. Proc Natl Acad Sci U S A. 2000.
Abstract
In over 90% of cervical cancers and cancer-derived cell lines, the p53 tumor suppressor pathway is disrupted by human papillomavirus (HPV). The HPV E6 protein promotes the degradation of p53 and thus inhibits the stabilization and activation of p53 that would normally occur in response to HPV E7 oncogene expression. Restoration of p53 function in these cells by blocking this pathway should promote a selective therapeutic affect. Here we show that treatment with the small molecule nuclear export inhibitor, leptomycin B, and actinomycin D leads to the accumulation of transcriptionally active p53 in the nucleus of HeLa, CaSki, and SiHa cells. Northern blot analyses showed that both actinomycin D and leptomycin B reduced the amount of HPV E6-E7 mRNA whereas combined treatment with the drugs showed almost complete disappearance of the viral mRNA. The combined treatment activated p53-dependant transcription, and increases in both p21(WAF1/CIP1) and Hdm2 mRNA were seen. The combined treatment resulted in apoptotic death in the cells, as evidenced by nuclear fragmentation and PARP-cleavage indicative of caspase 3 activity. These effects were greatly reduced by expressing a dominant negative p53 protein. The present study shows that small molecules can reactivate p53 in cervical carcinoma cells, and this reactivation is associated with an extensive biological response, including the induction of the apoptotic death of the cells.
Figures
Figure 1
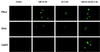
Nuclear accumulation of p53 in HeLa, SiHa, and CasKi cells by confocal immunofluorescence microscopy. The cells were treated with indicated concentrations of LMB and AD for 24 h. p53 was detected with DO-1.
Figure 2
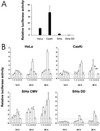
(A) Endogenous transcriptional activation of the p53 responsive promoter PG13 and MG15 containing mutated binding site for p53. The values represent relative activities of PG13 versus MG15 construct. SiHa DD is a SiHa cell line stably transfected with a dominant negative p53 gene. (B) Transcriptional activation of p53 responsive promoter after treatment with LMB and AD. Twenty-four hours after the transfection, cell lines were treated with the drugs and thereafter analyzed for luciferase activity. The bars represent: 1, 20 nM LMB; 2, 200 nM LMB; 3, 5 nM AD; 4, 50 nM AD; 5, 20 nM LMB + 5 nM AD; 6, untreated cells. Values are expressed relative to the readings of untreated cells arbitrarily set to 1. SiHa DD values are calculated relative to untreated SiHa CMV cells. * and **, not enough living cells for reliable quantitation. SD was calculated from triplicate wells.
Figure 3
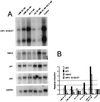
(A) Northern blot analysis of HPV 18 E6-E7, Hdm2, p21, and p53 mRNA expression in HeLa cells after 18 h of LMB and AD treatment. Each lane contained 3 μg of polyadenylated RNA. (B) Quantitation of the mRNA levels after normalization for GAPDH mRNA expression. The values are reported as the relative level over untreated cells.
Figure 4
Effect of LMB and AD treatment on the p53, p21, Hdm2, PARP, and p73 proteins. Western blot analysis of cell extracts from cultures treated for 18 h with 20 nM LMB (1), 200 nM (2), 5 nM AD (3), 50 nM AD (4), 20 nM LMB + 5 nM AD (5), and untreated cells (6).
Figure 5
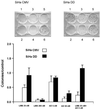
(A and B) Flow cytometry analysis of apoptosis in HeLa cells after 16 h of treatment with the combination of 20 nM LMB + 5 nM AD. (C) Hoechst 33258 staining of untreated HeLa cells. (D) Nuclear fragmentation of HeLa cells after 18 h of 20 nM LMB + 5 nM AD. (E) Western blot analysis of PARP protein in HeLa cells 35 h after 20 nM LMB + 5 nM AD (first lane) and without treatment (second lane).
Figure 6
Comparison of clonogenic cell survival of SiHa cells either stably transfected with empty vector (SiHa CMV) or with dominant negative p53 (SiHa DD). The treatments were 20 nM LMB (1), 200 nM (2), 5 nM AD (3), 50 nM AD (4), 20 nM LMB + 5 nM AD (5), and untreated cells (6).
Similar articles
- Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dormant tumor suppressor pathways.
Goodwin EC, DiMaio D. Goodwin EC, et al. Proc Natl Acad Sci U S A. 2000 Nov 7;97(23):12513-8. doi: 10.1073/pnas.97.23.12513. Proc Natl Acad Sci U S A. 2000. PMID: 11070078 Free PMC article. - Neocarzinostatin induces an effective p53-dependent response in human papillomavirus-positive cervical cancer cells.
Bañuelos A, Reyes E, Ocadiz R, Alvarez E, Moreno M, Monroy A, Gariglio P. Bañuelos A, et al. J Pharmacol Exp Ther. 2003 Aug;306(2):671-80. doi: 10.1124/jpet.103.051557. Epub 2003 May 15. J Pharmacol Exp Ther. 2003. PMID: 12750435 - An inhibitor of nuclear export activates the p53 response and induces the localization of HDM2 and p53 to U1A-positive nuclear bodies associated with the PODs.
Laín S, Midgley C, Sparks A, Lane EB, Lane DP. Laín S, et al. Exp Cell Res. 1999 May 1;248(2):457-72. doi: 10.1006/excr.1999.4433. Exp Cell Res. 1999. PMID: 10222137 - A cyano analogue of boswellic acid induces crosstalk between p53/PUMA/Bax and telomerase that stages the human papillomavirus type 18 positive HeLa cells to apoptotic death.
Khan S, Chib R, Shah BA, Wani ZA, Dhar N, Mondhe DM, Lattoo S, Jain SK, Taneja SC, Singh J. Khan S, et al. Eur J Pharmacol. 2011 Jun 25;660(2-3):241-8. doi: 10.1016/j.ejphar.2011.03.013. Epub 2011 Apr 2. Eur J Pharmacol. 2011. PMID: 21440536 - Induction of p53, p21 and apoptosis by silencing the NF90/NF45 complex in human papilloma virus-transformed cervical carcinoma cells.
Shamanna RA, Hoque M, Pe'ery T, Mathews MB. Shamanna RA, et al. Oncogene. 2013 Oct 24;32(43):5176-85. doi: 10.1038/onc.2012.533. Epub 2012 Dec 3. Oncogene. 2013. PMID: 23208500 Free PMC article.
Cited by
- Knock down of p53 or its ubiquitin ligase E6AP does not affect the sensitivity of human papillomavirus-positive cervical cancer cells to cisplatin.
Michnov O, Solomayer E, Fehm T, Stubenrauch F, Iftner T. Michnov O, et al. Am J Cancer Res. 2012;2(3):309-21. Epub 2012 Apr 22. Am J Cancer Res. 2012. PMID: 22679561 Free PMC article. - Cancer cells activate p53 in response to 10-formyltetrahydrofolate dehydrogenase expression.
Oleinik NV, Krupenko NI, Priest DG, Krupenko SA. Oleinik NV, et al. Biochem J. 2005 Nov 1;391(Pt 3):503-11. doi: 10.1042/BJ20050533. Biochem J. 2005. PMID: 16014005 Free PMC article. - Leptomycin B enhances CDDP-sensitivity via nuclear accumulation of p53 protein in HPV-positive cells.
Naniwa J, Kigawa J, Akeshima R, Kanamori Y, Itamochi H, Oishi T, Iba T, Terakawa N. Naniwa J, et al. Cancer Sci. 2003 Dec;94(12):1099-103. doi: 10.1111/j.1349-7006.2003.tb01406.x. Cancer Sci. 2003. PMID: 14662026 Free PMC article. - Pitx2a expression alters actin-myosin cytoskeleton and migration of HeLa cells through Rho GTPase signaling.
Wei Q, Adelstein RS. Wei Q, et al. Mol Biol Cell. 2002 Feb;13(2):683-97. doi: 10.1091/mbc.01-07-0358. Mol Biol Cell. 2002. PMID: 11854422 Free PMC article. - Nordihydroguaiaretic acid inhibits growth of cervical cancer SiHa cells by up-regulating p21.
Gao P, Zhai F, Guan L, Zheng J. Gao P, et al. Oncol Lett. 2011 Jan;2(1):123-128. doi: 10.3892/ol.2010.205. Epub 2010 Nov 10. Oncol Lett. 2011. PMID: 22870140 Free PMC article.
References
- zur Hausen H. Biochim Biophys Acta. 1996;1288:F55–F78. - PubMed
- Park D J, Wilczynski S P, Paquette R L, Miller C W, Koeffler H P. Oncogene. 1994;9:205–210. - PubMed
- Scheffner M. Pharmacol Ther. 1998;78:129–139. - PubMed
- Scheffner M, Werness B A, Huibregtse J M, Levine A J, Howley P M. Cell. 1990;63:1129–1136. - PubMed
- Kubbutat M H, Vousden K H. Trends Microbiol. 1998;6:173–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous